Project Grant 2017
The Achilles’ heel of breast cancer
Principal investigator:
Dr. Anita Göndör
Co-investigators:
Jonas Bergh
Lars Holmgren
Rolf Ohlsson
Jesper Tegnér
Institution:
Karolinska Institutet
Grant en SEK:
SEK 36.8 million
Tumor cells can invade surrounding healthy tissue, survive in the blood stream, and form metastases in other organs in the body. Quite simply, they are extremely good at adapting to different environments. Cancer cells have a greater ability to change than normal cells, which enables them to survive and develop in changing conditions.
This adaptability makes cancer cells dangerous. But this very adaptability distinguishes cancer cells from healthy cells, and so could also be used as a therapeutic target against them. This strategy is now being studied in a project at Karolinska Institutet (KI), funded by the Knut and Alice Wallenberg Foundation.
“Unfortunately, since current cancer therapies do not target the most characteristic feature of a tumor – its ability to adapt – tumors can develop resistance to most treatments. We want to identify the mechanism driving this adaptability, so we can develop new ways of treating cancer,” says Anita Göndör.
They believe the explanation lies in changes in the way DNA is organized in the cell nucleus.
Waking silenced genes
DNA in the cell nucleus is folded in a complex known as chromatin. Chromatin containing active genes tends to localize in the interior of the nucleus. Gene regions that are repressed, however, tend to be found at the outer edge of the nucleus, in an environment that inhibits gene activation.
This separation is important so that the cell can maintain its characteristics acquired during normal development. But in cancer cells the system is disrupted. There is greater contact between inactive and active regions, and the mechanisms the cell uses to maintain its characteristics – its epigenetic state – are destabilized.
“The cell suffers from a loss of memory. Our hypothesis is that this enables cancer cells with various characteristics to emerge, which then drive tumor development.”
It has recently been shown that activation of genes that are normally only turned on in early embryonic cell stages, is linked to the emergence of immature, stem cell-like tumor cells that can drive tumor development.
The researchers at KI want to find out whether this unscheduled gene activation can be explained by the reorganization of chromatin at the outer edge of the cell nucleus. They believe this may be due to changes in the cell environment caused by inflammation and ageing for example.
Better treatment of breast cancer
One aim of the project is to identify factors in the three-dimensional organization of DNA that drive the changeable behavior of cancer tissue. The researchers hope to find markers for cancer cell plasticity that can be used to classify the behavior and prognosis of tumors more accurately.
“We hope to learn to better predict how the tumor will behave in the patient, to predict aggressiveness and the risk of resistance to treatment. This would be particularly useful for an early form of breast cancer where we know that some patients will develop a more advanced disease. At present we have no methods of identifying them.”
The researchers also want to study how the adaptability of cells helps them to elude chemotherapy. Tumor cells have the ability of becoming dormant, remaining in that state for several years without dividing. Chemotherapy targets dividing cells, so they escape treatment in this way, before being reactivated much later on, causing metastases.
Little is currently known about this dormant state, and how cells can enter it and leave it. This is something the researchers at KI want to change.
“Our hypothesis is that although the cells are dormant, they undergo some kind of evolution, becoming increasingly plastic. We will characterize the three-dimensional organization of DNA in the dormant phase, to understand the part it plays in cell state change, and whether there is any way of preventing it.”
Broad expertise needed
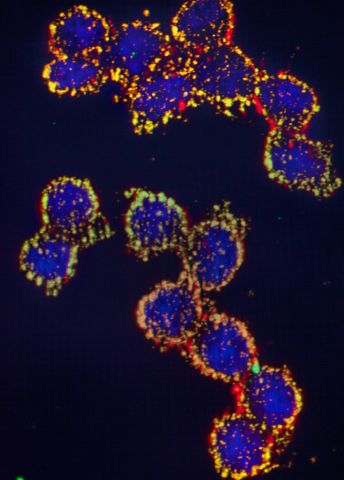
Göndör pores a while over an image on the microscope screen. The image shows specific protein complexes that regulate how one part of DNA interacts with another, distant, region at the periphery of the nucleus.
Methods of mapping the three-dimensional structure of DNA in various ways are crucial to the project. Members of the research team have developed techniques for mapping of three-dimensional genome organization in small cell populations and individual cells; techniques which are needed to analyze tumor material.
The researchers’ approach is to adopt a holistic view of the cell, and explore how its regulatory layers communicate with each other. The team therefore includes researchers knowledgeable in various aspects of cancer development. The researchers possess clinical skills, along with expertise in fields such as chromatin, biomechanical signaling, cell metabolism, and not least advanced computational biology, needed to analyze all the data that will be generated.
Göndör describes the project with enthusiasm. She points out that no one has previously mapped in a systemic manner the changes occurring in the three-dimensional organization of DNA in cancer stem-like cells. The long-term funding of the project gives the team the courage to try.
“The funding enables us to adopt a broader approach – to ask research questions involving a high level of risk and potential rewards. The answers may have hugely innovative implications.”
Text Sara Nilsson
Translation Maxwell Arding
Photo Magnus Bergström