Project Grant 2017
Understanding malaria-parasite survival in the human body for developing antimalarial drugs
Principal investigator:
Mats Wahlgren, professor in parasitology
Co-investigators:
Karolinska Institutet
Ulf Ribacke
Uppsala University
Suparna Sanyal
Lund University
Johan Flygare
Institution:
Karolinska Institutet
Grant in SEK:
27.5 million over five years
Malaria is caused by unicellular parasites of the Plasmodium genus, and is common in tropical regions. Every day about half a million people are infected with malaria by mosquito bites. Wahlgren, who is Professor of Parasitology at Karolinska Institutet (KI), elaborates:
“About 15,000 of those people become dangerously ill, which means they may end up in a coma, experience breathing difficulties or suffer from a serious blood shortage, and die.”
Wahlgren and his research team are housed on the ninth floor of the Biomedicum building on the KI campus in Solna, just north of Stockholm. In the lab they are trying to understand the malarial infection process, and are looking for candidate molecules for new drugs.
“The fact that malaria mainly affects poor people spurs me on in my research.”
Curing malaria is hindered by the parasites’ enormous ability to adapt and develop resistance to the antimalarial drugs currently available. They can survive in the body for long periods – up to two years. Wahlgren explains:
“By the time the immune system has caught up with the parasite and tries to kill it, it has changed the molecules on its surface. This makes it difficult to find variants of the parasite for use as a vaccine, for example.”
Spotlight on severe malaria
The main goal of the research is to understand the most dangerous form of malaria. Wahlgren explains how infection starts with a bite from a female Anopheles mosquito. Before it begins sucking blood, it injects saliva to prevent the blood from coagulating.
“The parasites are present in the mosquito’s saliva glands. They reach the liver via blood circulation, and grow there for two weeks or so. They then enter the blood, rupturing liver cells and destroying red blood cells.”
In the 1980s Wahlgren discovered one of the mechanisms enabling the most common malaria parasite – Plasmodium falciparum – to cause severe malaria. This happens when red blood cells infected with malaria form clumps, blocking the blood vessels, a phenomenon known as “resetting”. He uses a short film on his computer to show what happens. The blood in the experiment came from a boy who died in Malawi.
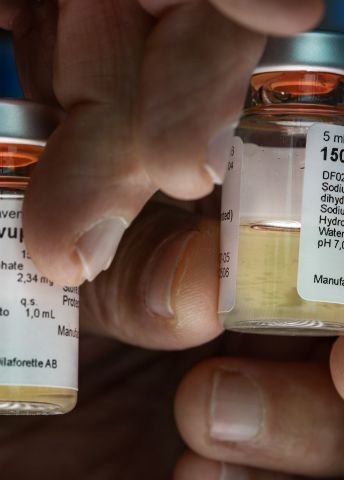
“We have been striving for many years to understand and map the molecular processes causing these severe pathological conditions. In collaboration with Modus Therapeutics, a company I started, we have also developed a drug that dissolves the blockages in the blood vessels. It has been tested in Thailand on people with mild malaria, and we want to go on and test it on people suffering from severe forms of the disease,” Wahlgren says, holding up a small vial labeled “Sevuparin”.
With funding from the Knut and Alice Wallenberg Foundation, Wahlgren and his colleague Ulf Ribacke are also pursuing a new promising line of research. It involves the cell biology of P. falciparum; they are looking for the parasite’s Achilles heel. The focal point of the studies is “translation”, i.e. how RNA (ribonucleic acid), in the parasite’s cells forms proteins. The project also includes research teams in Uppsala and Lund.
“We think the reasons the parasite develops resistance are largely to do with translation. We hope to find targets so we can block translation by the parasite, thereby killing it,” Wahlgren explains.
Thanks to the project grant, they have been able to invest in their own sequencing machine. The machine speeds up mapping of the building blocks of RNA, enabling the researchers to identify new targets for study.
Speeding up ribosomes
The parasites used in the research are cultured in the lab using human blood from the blood bank. The next step is to remove the ribosomes – the cells’ “protein factories”– or RNA, and study how proteins are formed by the parasite.
“We then examine how we can block translation using different drug molecules, and check that it does not impair healthy human cells. It’s a time-consuming process, and you need a fundamental understanding to find the right targets for study,” comments Wahlgren.
One important discovery is a protein that speeds up the ribosomes so they translate more. The protein has been found in parasites embedded in the placenta of pregnant women infected with malaria, a key target group for new drugs. Some 50 million pregnancies a year involve women who bear the disease.
There are a number of major challenges facing malaria research. One is the absence of an animal model. New drug molecules cannot be tested on rats or mice because the parasite cannot survive in these animals.
Wahlgren hopes nonetheless that in a few years’ time some candidate drugs will have been developed that are capable of inhibiting protein formation by the parasite.
“But it’s a long road, requiring patience and imagination, since we are doing things that no one has done before. The funding we have received is hugely important. A sizeable long-term grant is needed to carry out a project of this kind.”
Text Susanne Rosén
Translation Maxwell Arding
Photo Magnus Bergström





