Claudia Mohr is studying atmospheric aerosols. They are small airborne particles originating from human activities such as traffic and factories, as well as natural processes. The aim is to improve our understanding of their chemical composition, how they impact clouds, and through them, climate.
Claudia Mohr
Associate Professor of Environmental Science
Wallenberg Academy Fellow prolongation grant 2022
Institution:
Stockholm University
Research field:
Experimental studies of the impacts of atmospheric aerosols on cloud formation and climate.
In spring 2018 Mohr spent two months in Bolivia, near the summit of the Chacaltaya mountain. The research station, at 5,240 meters above sea level, is the highest atmospheric monitoring station in the world.
“Since the research station is so high, it records the influence of air masses coming from the Amazon rain forest, as well as from the city La Paz. I monitored the chemical composition of airborne particles and gases to gain a better understanding of the sources of these two types of pollutant and also their impact,” Mohr says.
So it is an interesting place to study the interaction in the atmosphere between emissions caused by humans and natural ones. And there are several reasons for monitoring emissions in South America.
“Environmental research is often carried out where the money is – in Europe and the U.S. But we also need monitoring sites in the southern hemisphere. Otherwise we won’t fully understand the global picture.”
Likes instruments
Mohr first got a taste for experimental environmental research during a placement at the Paul Scherrer Institute in Switzerland, her home country. She was studying environmental science, and wasn’t sure what she would do after graduating.
“It was great to see all the instruments, and the experiments and monitoring that could be performed with them. I loved it.”
The environment was a common topic of conversation at home when she was growing up. Her father worked at the Swiss environmental office, and her mother had been a lab technician at a chemistry laboratory.
“In a way, I have always been fascinated by environmental science and atmospheric science, and by how the world works. Everything is so interdependent. A tiny particle released at one end of the planet can be carried by the wind to an entirely different place, or end up in soil or water. We humans have a huge responsibility for what we are doing to our environment.”
“The grant is a fantastic opportunity for a young researcher. It will give me the scope and the freedom to realize my ideas and go where my curiosity leads me.”
Clouds key to climate
Atmospheric aerosols contain many different molecules and compounds. Mohr explains:
“I use advanced instruments to study minute details of the particles, while also attempting to address major issues such as climate change and air quality.”
Aerosol particles are needed so that clouds and raindrops can form, and clouds have a major impact on global climate. They form part of the hydrological cycle, but they can also reflect sunlight so that it does not reach the Earth’s surface, and stop heat escaping from the Earth. Mohr will examine the particles that end up in clouds – where they come from, and how they change.
“Atmospheric aerosols influence the properties of clouds, which is where we humans come into the picture. Our emissions impact particles and clouds, and by that the climate. This interaction still remains extremely difficult to understand and quantify in the analysis of the climate change.”
Collaboration essential
As a Wallenberg Academy Fellow, Mohr now has the opportunity to build her own experimental atmospheric research team at Stockholm University.
“The research performed here complements what I want to do very well. At the unit for atmospheric science we collaborate with researchers who model aerosols and cloud formation. They need experimental data to put in the models, and we need the models to obtain the bigger picture.”
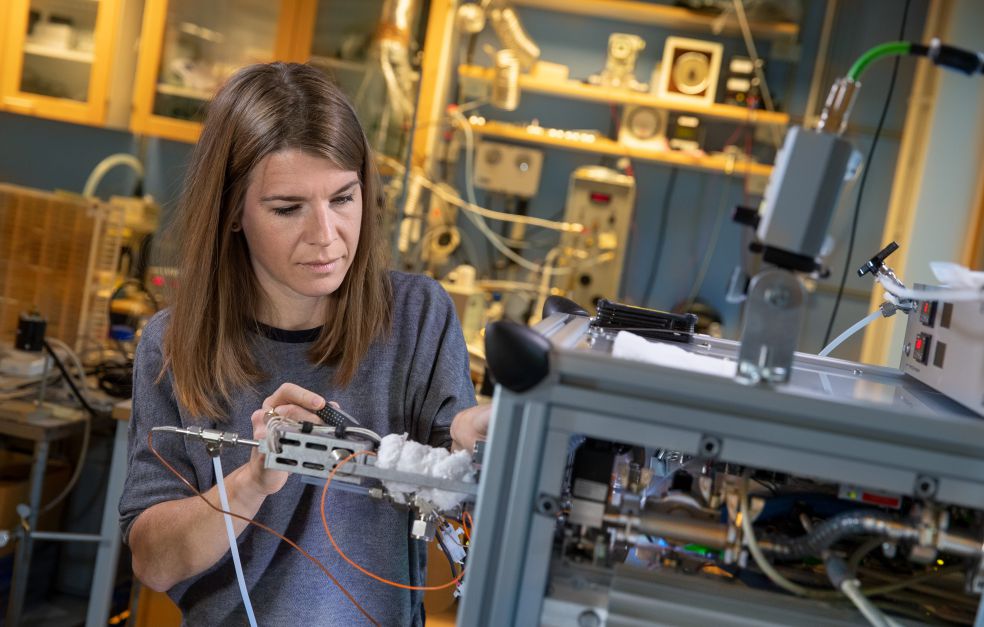
In the laboratory stands the group´s newly acquired mass spectrometer. It’s fairly heavy but can be used in the field. Mohr beams with pleasure as she shows how it works.
“Mass spectrometry is a traditional method for chemical analysis. But it’s a little different for those of us conducting aerosol research – we’re trying to analyze small particles in the air, preferably in situ. This instrument is fairly new for particle analysis, and it enables us to study gases as well. We can study almost everything present in the air, which opens up a multitude of possibilities in our research.”
The team plans to take the instrument with them to Delhi in India to learn more about the current air pollution situation, and what makes the air quality there so poor. In summer 2019 they will be heading off to Svalbard in the Arctic. Mohr says that field work is always a challenge:
“The instruments are really good, but that also makes them very sensitive. They’re like racehorses – very nervous, and in need of tender loving care. There is always a great risk that we won’t get the readings we need. And nature can be capricious. We could travel somewhere to monitor clouds, only to find that it’s a dry summer, with a cloudless sky.”
There are many uncertainties in attempting to predict future climate scenarios. It’s a highly complex task. Mohr sums up:
“All the knowledge and information I can contribute may be of help in this process. The work feels meaningful, which drives and motivates me.”
Text Susanne Rosén
Translation Maxwell Arding
Photo Magnus Bergström