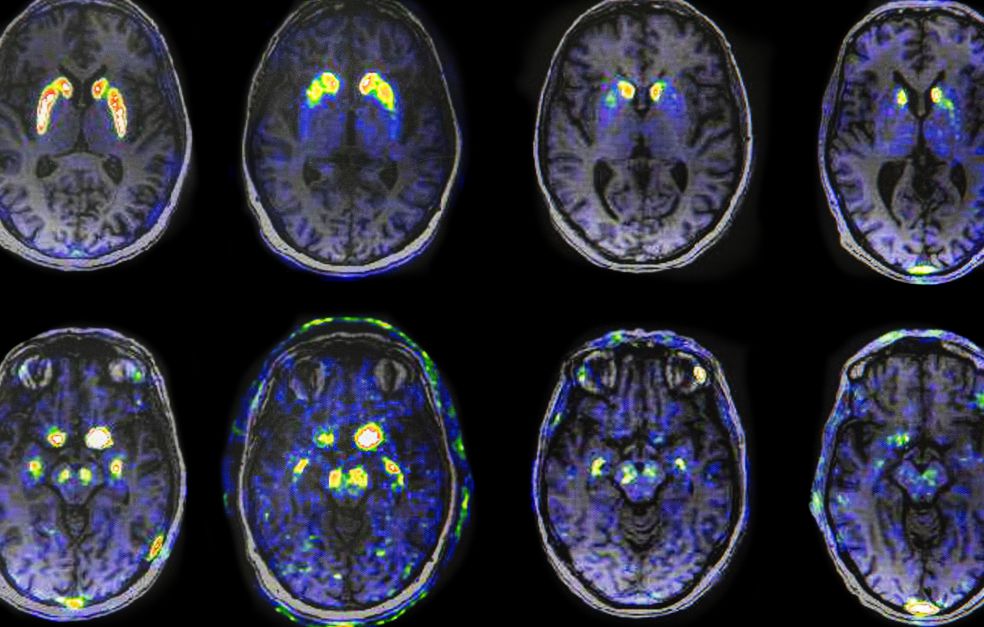
Many of Per Svenningsson’s patients have been diagnosed with Parkinson’s disease. But their symptoms and the rate at which the disease progresses vary a great deal. Svenningsson is studying the proteins and biological mechanisms involved in the development of the disease. The aim is to tailor therapies capable of slowing it down.
Per Svenningsson
Professor and Senior Consultant in Neurology
Wallenberg Clinical Scholar, prolongation grant 2021
Institution:
Karolinska Institutet
Research field:
Proteins playing a central role in the development of Parkinson’s disease
Svenningsson sees most of his patients at the Center for Neurology in Solna, just north of Stockholm. The center is an academic specialist unit for MS and Parkinson’s, and is run jointly by Karolinska Institutet, Karolinska University Hospital and Region Stockholm.
“It’s a huge privilege to be able to work in this way. We have a group of about 700 patients whom we are monitoring very closely by identifying various Parkinson’s symptoms, and by collecting genetic data, spinal fluid, plasma and immune cells.”
Subject to patient approval, the information gathered is used to study biological mechanisms and potential therapies in the research lab at Karolinska University Hospital.
“We want to learn more about how Parkinson’s disease develops in general, and how it develops in different people at cell and molecular level. The goal is to tailor therapies capable of slowing the progress of specific sub-categories of the disease.”
Parkinson’s disease is a progressive disease in which the nerve cells that produce the neurotransmitter dopamine gradually die. In most cases the disease advances slowly. Svenningsson has been seeing a high proportion of his patients for many years, and has got to know them well.
Dopamine plays a central role in the brain’s control of bodily movements. As dopamine levels drop, symptoms occur in the form of slower movements, stiffness and tremors. Many Parkinson’s sufferers also experience sleep problems, depression and dementia. Age is the number one risk factor for Parkinson’s disease. Onset is most common among people in their sixties, but the disease can also strike much earlier. There are slightly more male sufferers than female.
“There are now drugs that can replace dopamine and alleviate early-stage symptoms, particular motor symptoms, but there are no drugs capable of slowing down the disease.”
“The Clinical Scholar scheme has been great for my research, and has created opportunities for clinicians to receive PhDs. It’s also an important acknowledgement of my research, which is highly gratifying.”
Lewy bodies disperse in the brain
Svenningsson explains that the death of dopamine-producing neurons in the brain correlates with formation of plaque, made up of the protein alpha-synuclein. This type of plaque is called Lewy bodies. These are found in the gastrointestinal tract, the brain stem and olfactory bulb, and spread throughout the brain as the disease progresses. Loss of smell, constipation, disturbed dream sleep and depression are early symptoms of Parkinson’s disease.
“The same early symptoms and Lewy bodies are seen in Lewy body dementia, the second most common form of dementia. The regions of the brain that are affected differ, but these diseases are closely related. In the early stages it can sometimes be unclear whether a person has Parkinson’s disease or Lewy body dementia.”
One goal of the Wallenberg Clinical Scholar project is to identify early disease markers. Ideally, the aim is to detect the disease before onset. The researchers also want to understand what causes the Lewy bodies to spread in the brain.
In most cases the disease is idiopathic, i.e. the cause is unclear, but there are also genetic variants. Svenningsson is studying a group of people who have inherited a specific mutation that causes a tenfold increase in the risk of developing Parkinson’s.
“Our patient cohort includes about 100 people with mutations in the GBA gene, which codes for a protein called glucocerebrosidase. Those with this mutation develop a more aggressive variant of Parkinson’s, with onset occurring closer to 50 than 60 years old. They also experience severe cognitive impairment.”
The research team has made great strides in the lab in their work on a highly interesting molecule they believe may be a drug candidate for treatment of patients with mutations in the GBA gene.
“We’ve seen from animal models that this molecule may act favorably against alpha-synuclein protein, i.e. Lewy bodies, and slow the progress of the disease. These findings are of great interest, but we want to gain a more detailed understanding of the mechanism before we move on to testing the molecule on patients in clinical studies.”
Paving the way for precision medicine
The researchers’ sights are set on developing inhibitor drugs, but there are a number of major challenges to overcome along the way. Svenningsson elaborates:
“Translating findings from animal experiments to patients is problematic. But we’re constantly improving our models and methods.”
Another major challenge is the slow progress of the disease, which necessitates resource-intensive studies over long periods to see the results of treatment.
“The wide variation in progress of the disease from one individual to another also makes the studies more difficult. Here, precision medicine may offer a way forward. Funding from Knut and Alice Wallenberg Foundation allows us more resources to monitor patients and find sub-groups that we can use as targets for specific therapies.”
Text Susanne Rosén
Translation Maxwell Arding
Photo Magnus Bergström