Project Grants 2011
Catalysis in selective organic synthesis
Principal investigator:
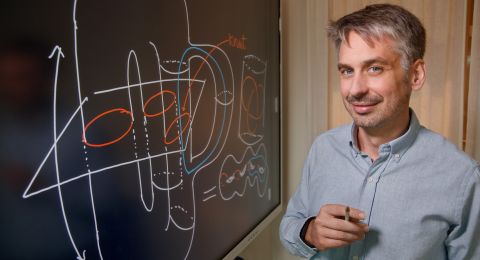
Jan-Erling Bäckvall, professor of organic chemistry
Co-investigators:
Hans Adolfsson
Kálmán Szabó
Belén Martín-Matute
Fahmi Himo
Per Siegbahn
Björn Åkerman
Xiadong Zou
Institution:
Stockholm University
Grant in SEK:
44 million over five years
Catalysts, such as metals and enzymes, have a unique ability to select and activate chemical compounds. Catalytic processes have long been known and are central to everything from chemical engineering to the function of the human body.
In recent decades our knowledge of the function of catalysts at the molecular level has grown dramatically, and developments in the field have been recognized in the form of multiple Nobel Prizes. This means that scientists today are much better equipped to develop new catalysts and catalytic systems and to govern the pathways for chemical reactions with precision.
“There’s an intra-disciplinary utility for what we do, as the methods we design are used by other organic chemists to bring about conversions in organic synthesis. And the possible benefits to society are clear. The methods we are developing can open new avenues to modern drugs for diseases like HIV, cancer, and Alzheimer’s,” explains Jan-Erling Bäckvall, professor of organic chemistry at Stockholm University and research director for a major catalysis project.
Three forms of catalysis
In the project Jan-Erling Bäckvall is working together with 25–30 colleagues from various research groups at Stockholm University. The scientists use both mechanistic studies and theoretical modeling to advance our knowledge of primarily three forms of catalysis: homogeneous catalysis, heterogeneous catalysis, and biocatalysis.
Homogenous catalysis involves the use of metals like palladium, ruthenium, and rhodium in homogeneous solutions. This type of catalysis experienced an upswing in the 20th century when a number of large-scale industrial processes were developed, including the Wacker process, Ziegler-Natta polymerization, and hydroformylation.
Heterogeneous catalysis also uses metals but in undissolved forms and sometimes bound on a support. This form of catalysis has been used for more than a century, for example in catalytic hydrogenation in order to convert liquid fats into solid fats for use as lubrication, etc. Heterogeneous catalysis is also the most commonly used form of catalysis in industry. Examples of practical applications include the Haber-Bosch process for producing ammonia and for catalytic exhaust purification in vehicles.
Chiral objects or systems
In the third form of catalysis – biocatalysis – researchers at Stockholm University are working with combinations of metal and enzyme catalysis. One important application for this type of catalysis is the production of chiral organic compounds. What characterizes these compounds is that the active molecules occur in two variants, enantiomers, which are exactly identical apart from the fact that they are mirror images of each other. In other words, it can be said that molecules relate to each other like a left and a right hand. In most cases the two variants evince different biochemical properties.
“The most common difference is that one of the variants has a higher effect, and in the worst case the other one, the less active one, can be outright harmful. One example is the anti-inflammatory drug Naproxen, where the undesirable variant of the active molecule causes liver damage. Chirality is also of great importance in other biological systems, such as pesticides in agriculture,” says Jan-Erling Bäckvall.
This problem can be solved in two ways: either by separating out the undesirable variant or by converting it into the desired variant. Jan-Erling Bäckvall and his colleagues are working with a method for converting the unwanted variant into the desired one. This doubles the yield and minimizes chemical waste compared with traditional methods.
"For our department this funding has been of tremendous importance, both for our economy and for our research volume."
Directed evolution
There is also a phase 2 regarding biocatalysis. It is what scientists call “directed evolution,” which can be likened to an accelerated breeding program for enzymes. In directed evolution, researchers deliberately alter certain parts of the genetic make-up of an enzyme. Then, with the aid of a number of different methods, they select out new variants of the enzyme that can be several hundred times more effective than the original variant of the enzyme
"A new world is opening up to us! Suddenly we can produce new catalysts via biocatalysis. We’re no longer locked into what nature makes available. Nature has taken millions of years to create optimal enzymes. Here we can do the same thing in the course of six months to a year," says Jan-Erling Bäckvall.
Besides the three forms of catalysis, these scientists are also working with electron transfer processes related to artificial photosynthesis.
“We’ve long attempted to reach such a comprehensive focus on catalysis, as we feel it’s a field with a future. We want the field to grow and we want to make major discoveries. But, of course, that’s something you can never predict,” concludes Jan-Erling Bäckvall.
Text Anders Esselin
Translation Donald S. MacQueen
Photo Magnus Bergström