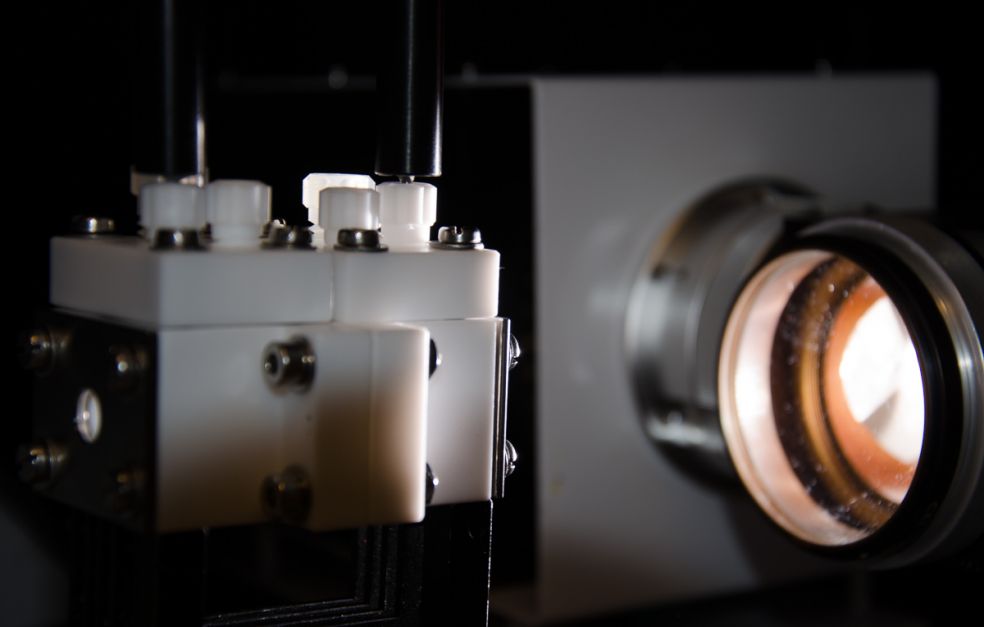
Haining Tian is enlisting the help of organic polymer chemistry to find sustainable alternatives to the fossil fuels of today. He aims to use nanoscale photocatalysts, known as “polymer dots”, water and carbon dioxide to convert solar energy into fuels. The goal is to generate hydrogen from water and produce carbon-based fuels from carbon dioxide in the atmosphere, thereby helping to mitigate the greenhouse effect.
Haining Tian
Associate Professor of Physical Chemistry
Wallenberg Academy Fellow, Prolongation grant 2024
Institution:
Uppsala University
Research field:
Polymer nano-photocatalysts for producing renewable solar fuels from water and carbon dioxide
“The main driver of my research is that we believe we can help to solve the energy problems we face. Sun is an abundant energy source all over the world. The question is how to convert it sustainably and efficiently into something else, like fuels,” says Haining Tian, who is an associate professor of physical chemistry.”
Tian has been interested in chemical conversion of solar energy since his PhD studies at Dalian University of Technology in China, an interest that led him to KTH Royal Institute of Technology and a postdoc position. Since 2014 Tian has built up his own research team at the Ångström Laboratory at Uppsala University.
“My background is in organic chemistry, so I always look for solutions to energy problems from that perspective. The research environment at the Ångström Laboratory is fantastic –
it’s an ideal base for me and my research. We have access to an advanced instrument park and collaborate closely with other research teams.”
There are many ways to convert the flow of energy from the sun. Tian elaborates:
“You can convert solar energy thermally into heat, or into electricity using solar cells. The aim of the project I am running with the support of Knut and Alice Wallenberg Foundation is to develop photocatalysts that can directly convert solar energy into hydrogen and carbon-based fuels using water and carbon dioxide. It is also about storing solar energy in fuels.”
“It’s really exciting. Thanks to the Fellow grant, I will be able to really concentrate on my research over a five-year period. I can recruit excellent researchers to work with in the project, and try to achieve my research goals.”
Eco-friendly polymer dots
Traditionally, the catalyst materials that have been used in conversion of solar energy has been metal-based materials. But in recent years the research field has seen a growing interest in catalysts based on organic polymers. Tian explains:
“If we convert solar energy into fuel using extremely costly or toxic metal-based materials, we miss our ultimate goal of creating completely renewable and sustainable systems. Therefore, we are working with organic polymers, which are not toxic. They are also degradable.”
One challenge posed by non-metallic catalysts is that they are not soluble in water and have limited reaction surfaces. Tian’s concept is based on tailored catalysts in the form of nanoscale polymer dots – Pdots, which are highly soluble in water. That trait increases the area of the reaction surface and the efficiency of the process.
Simulating sunlight
Tian envisions a photoelectrochemical component that can be placed inside a reactor, where it can both split water and perform carbon dioxide reduction without any other chemicals.
“The process is extremely environmentally friendly, since we only need to use water as a solvent. Our aim is to reduce carbon dioxide from the atmosphere. But in the lab we are using concentrated CO2 to carry out experiments and test the concept,” Tian says.
A major challenge is how to achieve a complete catalytic reaction. Tian explains that this means that when water is added to polymer catalysts the aim is to use sunlight to split the water molecules into oxygen and hydrogen. Usually, however, these polymers only work for one part of the splitting process. And there have so far been few studies on polymers that are both capable of splitting water and performing a full carbon dioxide reduction.
“We’ve been successful in producing hydrogen efficiently. We haven’t performed water oxidation to oxygen and carbon dioxide reduction before, so we’re now including that. When we’ve developed efficacious polymers for the various reactions, we can combine them, and create a system for performing complete reactions.”
Several steps remain
The catalytic process takes place at molecular scale, and Tian is using various spectroscopic methods and other techniques to study exactly what happens.
“The polymer dots are produced in the lab. We can tailor them so they are capable of absorbing different wavelengths of sunlight. By using simulators that generate sunlight and a reactor containing our polymer solutions we study the chemical reaction. We shine light on the reactor for a given length of time, this produces a gas, which we study using gas chromatography. The grant has enabled us to buy our own gas chromatography machine. It’s a key instrument for us.”
Several additional steps remain before it will be possible to produce renewable solar fuels from water and carbon dioxide alone, but the knowledge being gained about organic polymer catalysts is an essential part of getting there. At some point in the future Tian is keen to start a company based on the research findings in the project.
“Our hope is that the technology we are developing will form part of a sustainable energy system. This will also require other needs to be met, such as the method being efficient and stable enough, and not too expensive. I’m not dealing with these issues at the moment, but they will have to be incorporated later on if these light-driven systems are to be brought to market.”
Text Susanne Rosén
Translation Maxwell Arding
Photo P-Cats, Sina Wrede, Mariia Pavliuk, Markus Marcetic