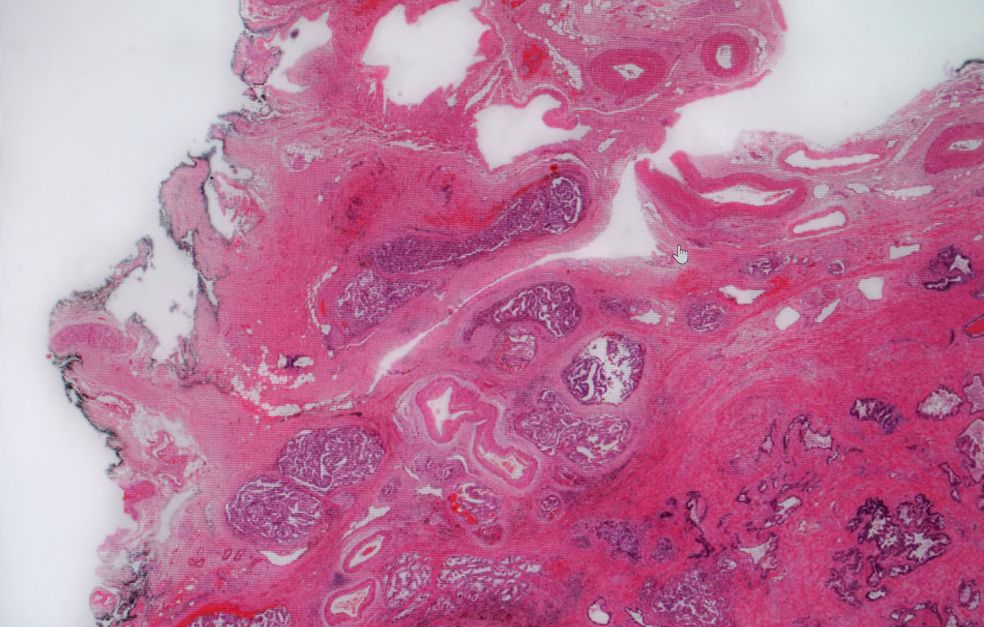
Prostate cancer is one of the commonest forms of cancer in Sweden. There are often no symptoms in the early stages, and the PSA diagnostic test is not completely reliable. Joakim Lundeberg is building on his earlier breakthroughs in gene analysis to create a more watertight diagnostic method.
Joakim Lundeberg
Professor of Gene Technology
Wallenberg Scholar
Institution:
KTH Royal Institute of Technology
Research field:
Translation of scientific findings in the field of spatial biology
Every year some 2,300 people in Sweden die of prostate cancer. This form of cancer accounts for one-third of all cancers in men, and is difficult to diagnose. The main diagnostic test currently in use is the PSA test, which involves taking a blood sample to measure the concentration of a certain protein produced in the prostate gland.
But an elevated PSA count does not necessarily mean prostate cancer. Levels may also be higher due to an enlarged prostate or in the aftermath of a urinary tract infection. Age also affects the results of a PSA test.
This form of screening is therefore considered to lead to overtreatment, and there is an urgent need for additional diagnostic methods.
“It’s essential to develop new biomarkers and methods so we can determine which patients will benefit most from different treatments. We need new methods capable of sparing many people from the side-effects of treatment that can have a huge impact on quality of life,” says Lundeberg, who is professor of gene technology at KTH Royal Institute of Technology.
His goal as a Wallenberg Scholar is to build on his earlier progress in the field of gene analysis together with new findings on prostate cancer in order to devise new diagnostic methods.
To date most researchers have searched for individual biomarkers for a given disease. But I think this approach is a little too simple; the body is much more complex. We are therefore searching for hundreds of biomarkers at the same time.
New prostate cancer findings
Over a decade ago Lundeberg and Professor Jonas Frisén developed a new technique for studying which genes are active in different tissues. The technology was given the name Spatial Transcriptomics, and was voted method of the year in the scientific journal Nature Methods in 2020.
“It’s now the most widely used method in the world for studying gene activity in tissue sections. We developed it by way of studies on prostate cancer, among other things,” says Lundeberg.
In later research, conducted jointly with researchers from the University of Oxford in England and elsewhere, the technique was used in a study of tumors and of the environment surrounding the prostate. This resulted in new findings about the early stages of prostate cancer.
“Surprisingly, we found multiple genetic changes in the environment outside the tumor. These were fantastic findings that gave us the idea of translating them into a broader diagnostic method for prostate cancer.”
A broad approach
Lundeberg’s research team is not mapping the changes that precede prostate cancer. Rather than studying genes individually, they include large blocks of DNA involving hundreds of genes at a time.
“While others search for a unique biomarker for a given disease, we are looking for hundreds at the same time. And we’ve already made a fair number of unique finds in these blocks that will be of use to us.”
The next step is to see which proteins are impacted by the large gene blocks. The changes result in expression of either more or fewer proteins. This change can be measured in the blood or preferably in urine, creating the simplest possible diagnostic method for future use.
These analyses have been made possible by developments in AI and machine learning over the past few decades. The team is also able to take advantage of technology from Olink Proteomics, a company based in Uppsala. They have developed highly sensitive reagents capable of identifying selected proteins in bodily fluids.
“One challenge is to cope with the enormous variation found among the proteins. But it’s an advantage in this context that we’re studying blocks of hundreds of genes at the same time, rather than individual molecules.”
More biomarkers
A parallel line of research is to determine tumor cell metabolism. Tumor cells release a number of smaller molecules to communicate with their surroundings. Lundeberg’s research team has developed a tool to identify the molecules leaving the cells.
“This is another method that can be developed into an earlier biomarker for prostate cancer than the PSA test. And here we can benefit from the knowledge gained from gene analysis.”
The findings may result not only in new diagnostic methods for prostate cancer, but also for breast cancer.
When Lundeberg appears in the foyer of the SciLifeLab building in Stockholm he apologizes for being jetlagged. The previous week he had attended a cancer conference in Australia, which brought together scientists researching both prostate and breast cancer.
“It was a unique opportunity, since these two fields so rarely meet. Scientific collaboration is often the key to progress. One of my strengths is bringing together people possessing the right skills to create synergies.”
SciLifeLab is a national research center serving as a base for research teams from four Swedish universities: Karolinska Institutet, KTH Royal Institute of Technology, Stockholm University and Uppsala University. One reason the research team began to study prostate cancer was the SciLifeLab research environment. When Frisén and Lundeberg took the first steps toward developing their technique they needed samples. Thomas Helleday in the next room just happened to be researching prostate cancer.
“SciLifeLab is a really important place. Being close to each other makes a huge difference, physically as well as organizationally. SciLifeLab is vital to our research.”
Text Magnus Trogen Pahlén
Translation Maxwell Arding
Photo Magnus Bergström