Project Grant 2018
The Birth of the Mitochondrial Ribosome
Principal investigator:
Alexey Amunts, Associate Professor
Co-investigators:
Karolinska Institutet
Martin Hällberg
Joanna Rorbach
Institution:
Stockholm University
Grant in SEK:
SEK 31,500,000 over five years
Amunts’ team has been awarded a five-year project grant by Knut and Alice Wallenberg Foundation to reveal parts of the cell’s universe at nanoscale.
“This new technology – cryogenic electron microscopy – enables us to see the mitochondrial ribosome and also to visualize the process of its formation” says Amunts.
Mitochondria are the power plants of our cells, converting food into biochemical energy that the cells can use. The proteins that do the actual work are synthesized in situ in mitochondria by special “protein factories” – mitochondrial ribosomes.
These differ from other ribosomes in the body in that they only translate the DNA code present in the mitochondrion itself. To understand what is so special about the mitochondrial ribosomes, Amunts and collaborators set to investigate how mitochondrial ribosomes become to be.
Explosive development
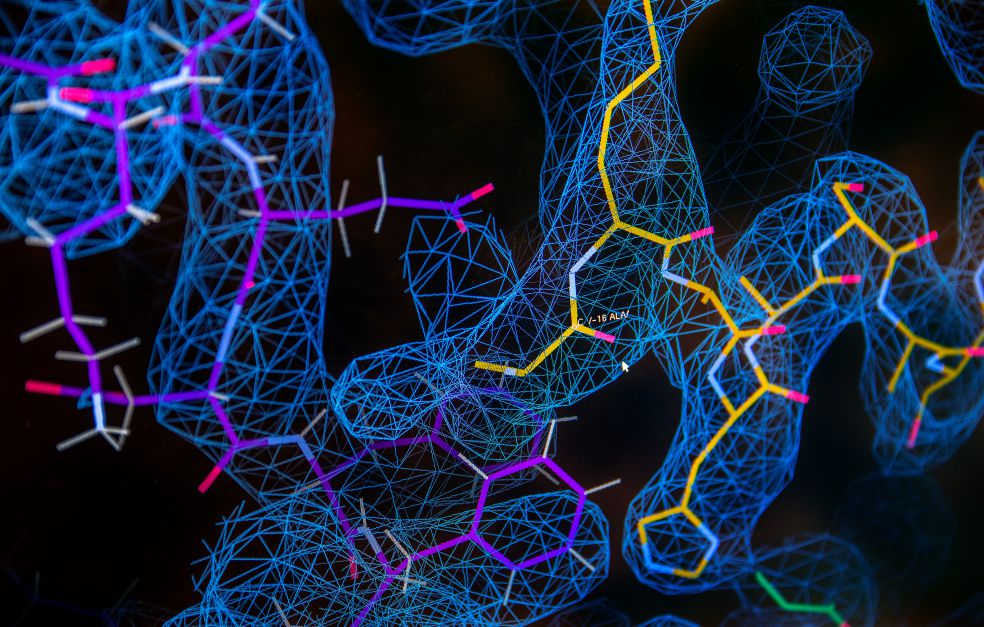
The path to understanding begins in a basement room at SciLifeLab in Stockholm. Members of Amunts’ research team are working here with one of Sweden’s two cryogenic electron microscopes. The scientists who originally developed cryogenic electron microscopy were awarded the Nobel Prize in Chemistry in 2017. The technique makes it possible to image molecules as three-dimensional structures, which is then interpreted into atomic models. This technique has enabled the life sciences to make huge strides in recent years.
“It’s an incredibly exciting time to be working in this field. Almost every day we gain insights into fundamental processes by examining them with the help of the new technique,” Amunts enthuses.
He came to do research in Sweden from a Nobel winning laboratory in Cambridge and now compares recent developments with the advent of microscopy in the late seventeenth century. It was then that the Dutch scientist Antonie van Leeuwenhoek developed the first microscope that was used to formulate the concept that organisms are made of cells.
“That technique enabled scientists to see the building blocks of living organisms. Now we can look at them from the inside at the nanoscale. What we see tells us how molecules are formed and how they work together to convert energy in mitochondria that sustains life.”
Both of Sweden’s cryogenic electron microscopes have received funding from the Wallenberg Foundation, and are now in such demand that a third is on its way to Stockholm.
Seeing proteins on the atomic scale
The work of the research team can be described as a multi-stage process. The mitochondrial ribosome molecules are first filtered out, before being fixed in water that has been frozen so quickly that it is more like glass – vitrified water. The sample is then bombarded with electrons in a microscope that captures the weak reflections from each molecule.
The result is thousands of images. These undergo highly advanced processing, combining them to form a three-dimensional map showing the positions of the building blocks in each molecule.
At the moment the sample is frozen the molecules are fully occupied with their tasks, such as adding an amino acid to a forming protein, releasing a completed protein chain or even building the mitochondrial ribosome itself. All these tasks can be distinguished and combined into an animated sequence of events, like a film.
“When we see the film, we can begin to consider how the different developmental stages fit together, and how they are linked.”
Huge quantities of data
The microscope generates enormous amounts of data, four terabytes a day, which are saved onto hard disks, and carried three floors up for analysis.
“These huge quantities of data make access to processing power all the more important,” Amunts says.
Although the computers’ smart algorithms perform the task of combining the images correctly, it is up to the researchers to use the information to build models of the molecules at atomic level and interpret the biological insight into how the mitochondrial ribosomes are formed to maintain the cellular energetic balance.
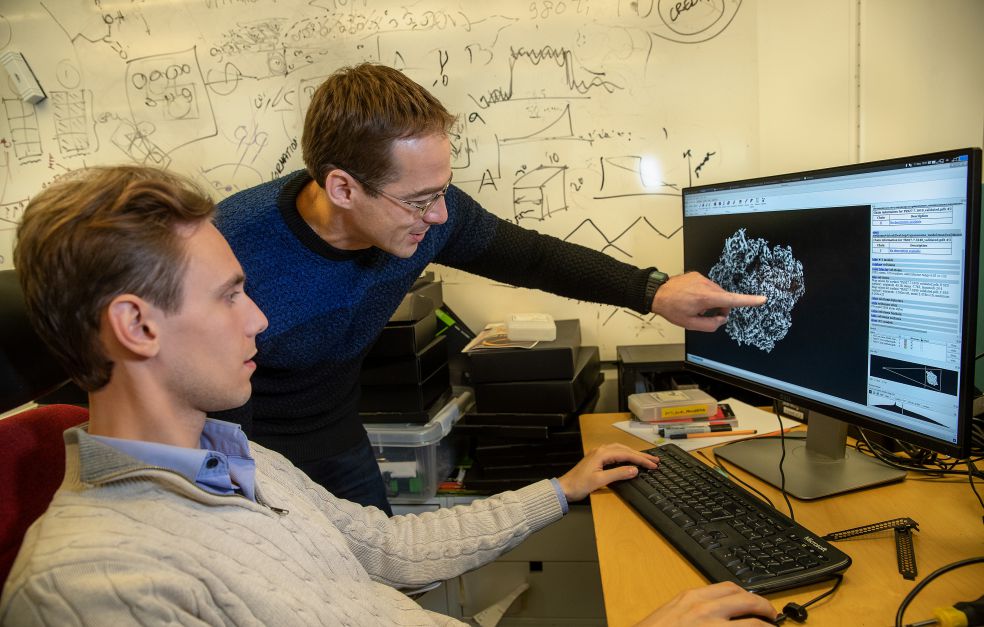
Rasmus Kock Flygaard is a postdoctoral member of the team. He is poring over information on the screen in front of him.
“We are building something that’s never been created before, so I begin with something resembling a sketch. I then retrace my steps time and time again to add the right parts to the final form,” he explains.
Amunts is standing behind his postdoc, and points eagerly at the screen.
“Here you can see a new structure of the mitochondrial ribosome assembly intermediate, an example of the fundamental discoveries we are making in the lab. These data have not yet been published, and the parts of the molecule you see appearing on the screen have never been seen before by anyone,” comments Amunts, and goes on:
“There is no more rewarding feeling for a structural biologist than seeing how molecules that we study are sculpted in keeping with the fundamental laws of biochemistry.”
Results already generated by the project include unexpected rapid progress in the field of synthesis of new proteins by the mitochondrial ribosome.
Amunts thinks the ambitious project would have difficulty to see the light of day without funding from Knut and Alice Wallenberg Foundation:
“The project’s five-year time span provides a unique opportunity to establish a collaborative effort to investigate new big questions that cannot be answered over a shorter time horizon.”
The availability of research funding was also one of the reasons he chose to move to Stockholm University from the renowned laboratory in Cambridge, where cryogenic electron microscopy has been advanced.
“The long-term funding enables us to attract very talented people and create an environment in which they can focus without having to be overly concerned with time or resources. The discoveries we are making are rooted in this culture and research freedom that the grant helps to create.”
Text Magnus Trogen Pahlén
Translation Maxwell Arding
Photo Magnus Bergström