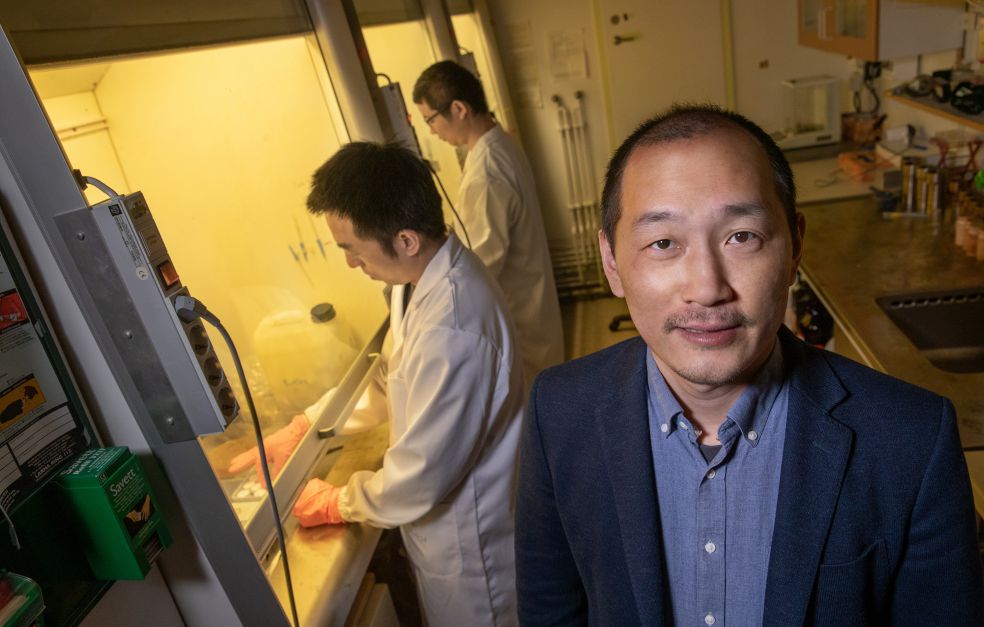
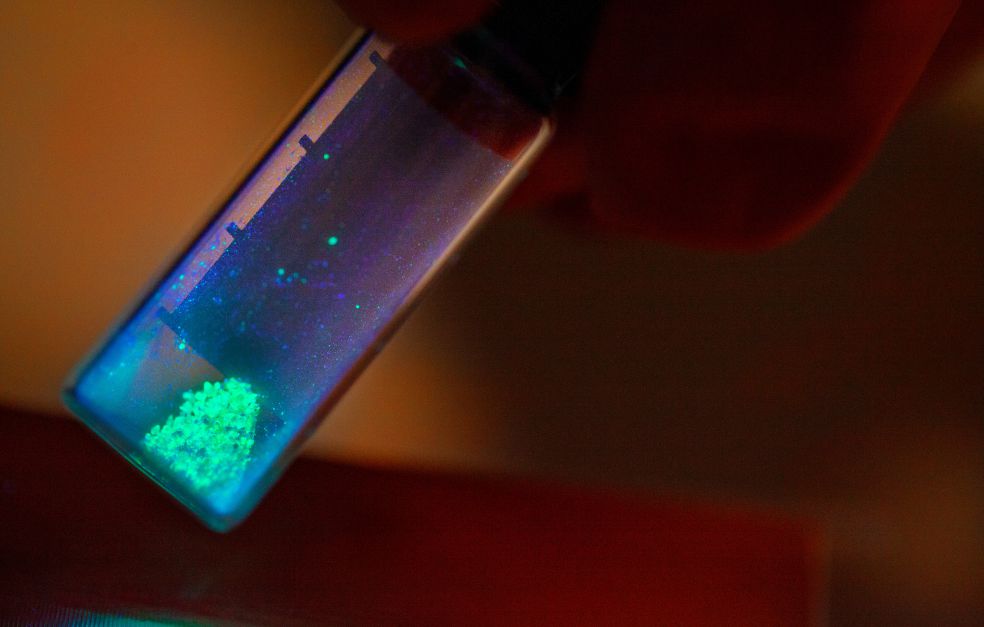
Crystalline materials of the perovskite type are easy to manufacture, and good at both absorbing and emitting light. These are perfect properties for solar cells and LEDs. But current perovskites are too unstable, and also contain lead. At Linköping University Wallenberg Academy Fellow Feng Gao is developing new methods of creating stable, non-toxic perovskites.
Feng Gao
Professor of optoelectronics
Wallenberg Academy Fellow 2017
Institution:
Linköping University
Research field:
Lead-free perovskites, perovskite LEDs and solar cells, organic solar cells
Perovskite is an umbrella term for all substances with a certain crystalline structure that gives them certain properties. In recent years there has been a growing interest in creating LEDs and solar cells using perovskite materials which possess an unusual ability to absorb and emit light. The silicon-based solar cells that currently dominate the market are capable of converting more than 20 percent of sunlight into electricity. The best prototype solar cells based on perovskites can reach a similar value. But there are a few major problems. The perovskites currently used are too unstable, and the most efficient ones contain lead. Lead is a heavy metal, and toxic to living organisms, and hardly a good component in a solar cell material to be made in the form of a paint applied to a surface.
“Lead is a critical issue to be resolved. We need to replace it with something more environmentally friendly. We can’t put materials containing lead on a roof, and run the risk of the lead leaching out via rainwater into the environment,” Gao says.
He is researching at Linköping University Department of Biomolecular and Organic Electronics, dividing his time between three areas: organic solar cells made of plastics, perovskite-based LEDs, and lead-free perovskites. Most of his work as a Wallenberg Academy Fellow will be on perovskites.
“Being chosen as a Wallenberg Academy Fellow means that I can focus on a high-risk idea for a relatively long time. It is also an important acknowledgement of my research, as the Foundation is so highly thought of in Sweden.”
Combining materials – an opportunity and a challenge
In traditional perovskites, lead forms ions with a charge of 2+. So far, most attempts to replace lead in perovskites have involved substituting it with another metal that forms ions having the same charge. But there are not many alternatives, and results have not been good. Instead, Gao wants to try replacing the lead ions with ions of two substances: half with a charge of 1+, half with a charge of 3+. This opens the way for far more options – so many, in fact, that their number is a problem in itself.
“We suddenly have an enormous library of combinations of materials to try out. This offers great potential, but also presents challenges. We have enlisted the help of experts on chemical calculations, who are helping us to narrow down the field to a shortlist of promising candidates. We will then synthesize crystals from those combinations and test their properties,” says Gao.
Energy efficiency is not the only issue. The new perovskites that Gao is developing are also more stable than their lead-based counterparts. Many of their other properties are not yet known. These are completely new materials, and analyzing their strengths and weaknesses is a central part of the work being done by Gao and his colleagues.
Pure light and broad absorption
Perovskites possess a number of properties that make them well suited for use in solar cells and LEDs. LEDs exploit the ability of the crystals to emit plenty of light, and light at different wavelengths. In addition, the light is very “pure”: light in red diodes is very red; in greed diodes it is very green. This strong, clear light is very well suited to displays and other applications. The color can easily be varied by substituting one substance in the crystal for another.
The solar cells benefit from the fact that perovskites absorb many of the sun’s wavelengths, and that electrons move freely in the material.
“I don’t think that perovskites will outcompete silicon-based solar cells. Although our materials may well be cheap, the price of silicon-based solar cells has also fallen dramatically. But I think we’ll see perovskites being combined with silicon to enhance efficiency – always presupposing that we solve the lead problem, of course.”
Research and entrepreneurialism in partnership
Gao received his bachelor’s and master’s degree in Nanjing, near Shanghai, and had begun to research even before he graduated. He then moved to Cambridge in the U.K. to study for his PhD. At that time he still had not decided whether he would commit to research in the long term. Cambridge is home to numerous start-ups, and Gao found himself becoming increasingly interested in entrepreneurialism and research. When he had received his PhD, he contacted Professor Olle Inganäs, a prominent solar cell researcher at Linköping University, asking to join his team as a postdoc.
“Here in Sweden, I discovered that Olle had started a number of companies. The businesses employ professional managers, so the scientists can continue their research and seek new knowledge. I realized that you can actually do both. It’s possible to make positive research findings, and see them put to practical use. This is something I really appreciate about the Swedish research environment.”
Text Lisa Kirsebom
Translation Maxwell Arding
Photo Magnus Bergström