
Project Grant 2011
Molecular solar energy science
Principal investigator:
Anders Hagfeldt, professor of physical chemistry
Co-investigator:
Stenbjörn Styring
Institution:
Uppsala University
Grant in SEK:
48 million over five years
One hour in the blazing sun won’t give you much of a summer tan. But that same measly hour would be enough to supply all of humanity with energy for a year – if we could just efficiently capture the sunlight that shines on the earth. All of the world’s energy problems could thus be solved in a flash if it were possible to convert and store solar energy without major losses.
Our hopes are on biomimetics, a technology that seeks to copy nature’s own smart mechanisms. A network of Swedish research teams at Uppsala University, KTH Royal Institute of Technology, and Stockholm University has been paving the way since the 1990s along multiple research tracks and is now a world leader.
Solar fuel directly from living organisms
One of the tracks is to improve upon nature’s own production of solar fuel. Cyanobacteria and green algae are photosynthetic microorganisms and are the only existing organisms that can use solar energy to produce hydrogen, which is a possible fuel for the future. The idea behind the project is to exploit genetic engineering and synthetic biology. With these methods, scientists can custom tailor energy metabolism to produce a maximum amount of fuel. Fuel from microorganisms can be extracted from special cultivation chambers where light is used to drive fuel production.
Our knowledge of enzymes from microorganisms is also used as a model for the next track: to mimic, in a purely chemical way, the photosynthesis of plants to produce fuel from water and sunlight.
“Natural photosynthesis, which is the basis of all life on earth, uses solar energy and water to produce energy-rich substances. We want to do the same thing, but in the laboratory, and produce a fuel like hydrogen instead,” Anders Hagfeldt, a professor of physical chemistry at Uppsala University.
To succeed, these scientists need to create supermolecules that can exploit the energy from the sun to split water and produce hydrogen gas, for example, just as green plants do.
It’s hard to produce hydrogen, but it’s possible, and at Uppsala researchers have devised a series of catalyzers that imitate the natural enzyme very well.
However, the great obstacle is to develop catalyzers that can oxidize water without also oxidizing themselves to pieces. This is also a big problem in nature. Chlorophyll is constantly being broken down and has to be recreated.
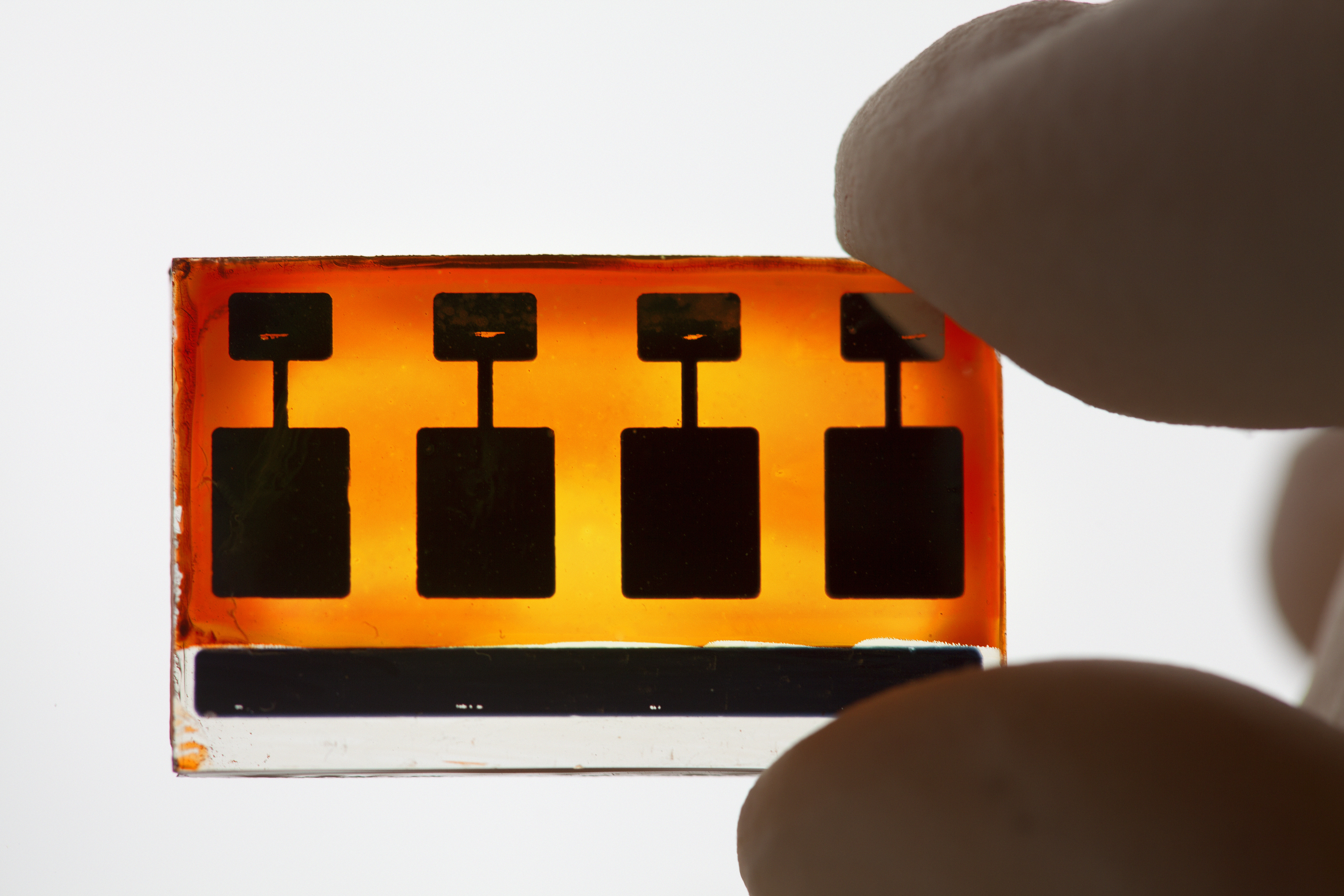
A new generation of solar cells
Both Uppsala and KTH have managed to develop promising catalyzers. At Uppsala they are based on the heavy metal cobalt, while Licheng Sun and his colleagues at KTH have succeeded in devising an efficient catalyzer with ruthenium that can go around more than 10,000 times before it breaks down.
In spring 2012 there was a new breakthrough, with a record fast catalyzer. Natural photosynthesis works with 100 to 400 exchanges per second. The new catalyzer can attain similar speeds, 300 exchanges per second, a substantial improvement considering that previous catalyzers reached a maximum of five exchanges per second.
“The results with the various catalyzers have attracted a great deal of attention around the world and are highly promising for the future,” says Anders Hagfeldt. “We’re the only network right now that has its own catalyzers that are driven by light both for splitting water and for making hydrogen.”
The third track is about developing more efficient solar cells that can store more energy. The focus is now on the next generation of solar cells, so-called Grätzel cells, which are extremely inexpensive. Ordinary glass is the most expensive component.
“The underlying principle is so simple that you can easily build a functioning solar cell at home, and we have even put together a lab package for use in chemistry classes in schools,” says Anders Hagfeldt.
The similarity to natural photosynthesis is that the solar energy is captured by a dye and that the charge is transported – in the Grätzel cell via a titanium dioxide membrane, instead of via a protein membrane as in plants.

Closer collaboration sparks new ideas
These Swedish research groups are now going to collaborate more closely in a project with support from the Knut and Alice Wallenberg Foundation. The hope is that this cross-disciplinary research will spawn new ideas and create synergies.
The driving force is a vision of solving the world’s energy problems. In the future private individuals will have a solar fuel facility on the roof at home, a sheet of glass where water is led in as a raw material and where hydrogen comes out the other side. Major solar power stations in sun-drenched areas or smaller facilities in cities and industries can become a reality. In this way, some areas can become self-sufficient and not nearly as vulnerable to outages or military conflicts.
“You can even imagine that we could convert solar energy not only into electricity and hydrogen gas but also to liquid fuels, which is advantageous since all the infrastructure is already in place. As researchers we have to dare to think boldly and strive for new breakthroughs. I really believe in solar energy as the great potential for supplying energy around the globe, even though it won’t be the only source of energy,” says Anders Hagfeldt.
Text Nils Johan Tjärnlund
Translation Donald S. MacQueen
Photo Magnus Bergström



