Diamonds form slowly under enormous pressure in the Earth’s interior. By mimicking the process in a supercomputer, Igor Abrikosov has managed to discover a dozen new metastable crystalline materials. He now wants to gain an in-depth understanding of their properties.
Igor Abrikosov
Professor of Theoretical Physics
Wallenberg Scholar
Institution:
Linköping University
Research field:
Theoretical physics, materials science, new metastable materials, quantum mechanics, theoretical simulations
Natural diamonds consist of carbon atoms compressed in the Earth’s mantle over millions of years. They are carried upward via lava flows, and as the lava solidifies, the diamonds become encapsulated in rock formations. Today they are mined from deep deposits in Angola, Botswana, Namibia, South Africa and elsewhere.
Diamonds are an example of a metastable material, characterized by high energy and existing in – or trapped in – relatively uncommon phases or states. This gives them useful properties as compared with the stable form of the material.
The stable form of carbon at normal atmospheric pressure is graphite. But why? Diamond is harder than graphite, after all.
“True, but the stable form doesn’t have to be the hardest. Many types of steel, for example, are metastable. The properties of a material depend on its energy or the strength of its atomic bonds,” explains Abrikosov. He is a materials researcher and professor of theoretical physics at Linköping University. His research involves use of theoretical simulations to synthesize and discover new and exciting metastable materials.
New diamonds
He aims to find “new diamonds” – new metastable materials that are synthesized in laboratories under high pressure and high temperature – but that also have the potential to be used at normal atmospheric pressure, which on Earth corresponds to one atmosphere or one bar.
During Abrikosov’s initial period as a Wallenberg Scholar, he developed the “recipes,” or quantum mechanics computations, to find new materials that then formed the basis for physical experiments.
These experiments have then been conducted by a number of researchers in laboratories, including physics professor Natalia Dubrovinskaia at the University of Bayreuth in Germany, who specializes in crystals.
Together, Abrikosov and his partners have succeeded in creating around fifty new metastable materials by mimicking the diamond formation process in experiments. However, most of them failed the transition from millions of atmospheres of pressure to normal atmospheric pressure.
But about a dozen managed to remain in a metastable state and survived under normal pressure.
“We have succeeded in creating new crystalline materials that are completely unknown to humankind,” says Igor Abrikosov, who wants to use his second Wallenberg Scholar period to explore these materials.
“We want to take control and force the material to assume a metastable state to achieve the desired properties.”
Once they are fully developed, the idea is to use these materials in applications such as electronics, tools, cutting machines and solar cells.
Journey to Uppsala
Abrikosov moved from Russia to Uppsala University in the early 1990s to conduct research as a postdoctoral fellow and since then has worked in the field of theoretical physics with application to materials science.
We have succeeded in creating new crystalline materials that are completely unknown to humankind.
Over the past five or six years he and his colleagues in Linköping have also studied how to exploit artificial intelligence in materials research. They are currently using the Berzelius supercomputer, also located in Linköping, to speed up their quantum mechanics simulations by a factor of a thousand.
“Thanks to the supercomputer, we might now be able to develop materials ready for applications in just five years,” he says, adding that they aim to select and focus on materials with the most interesting properties at one atmosphere.
An incredible feeling
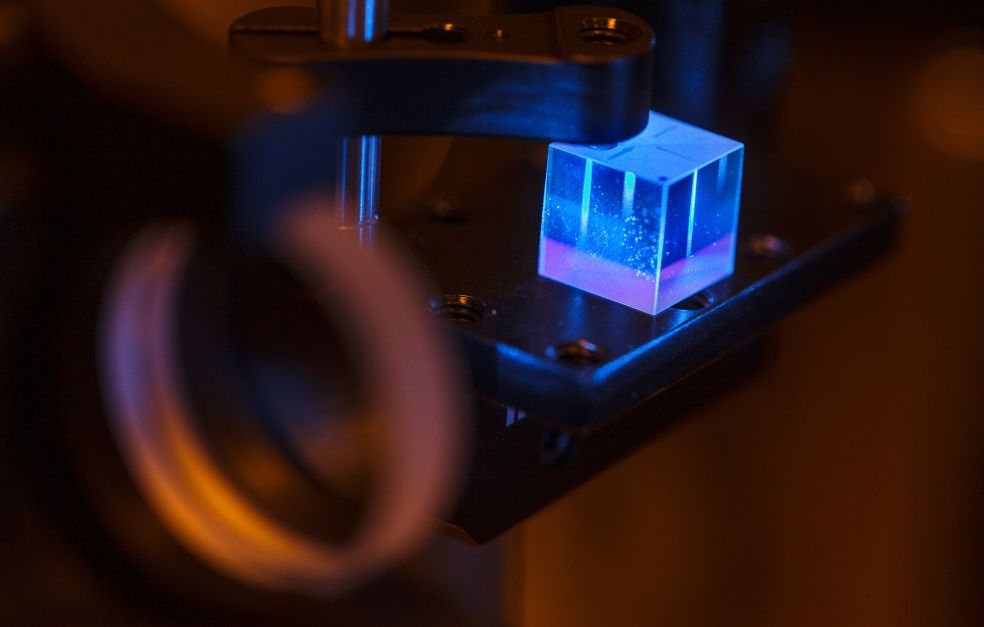
To create new metastable materials, in this case ultra-hard carbon nitrides, the researchers are using two diamonds whose tips mechanically compress carbon and nitrogen inside a circular gasket made of rhenium metal and filled with nitrogen gas.
A pressure of one million atmospheres is applied to the tips, and researchers raise the temperature to around 2,500 degrees Celsius using lasers.
A chemical reaction occurs, creating a minute amount of a new material made of carbon and nitrogen in the form of an ultra-hard crystal. However, it is extremely small – only one micrometer, i.e., one-thousandth of a millimeter.
“But it was still an incredible feeling when I held the first new material in my hands,” says Abrikosov, smiling.
The research team is now attempting to scale up the extremely small materials to roughly the size of a diamond.
Among other things, they are testing a “large volume press”, a device used in modern synthetic diamond production. However, one challenge is that it probably cannot create the necessary high pressures.
Another method, currently used to grow synthetic diamonds, is to add chemicals.
“But first we need to find the right chemical that can trigger similar growth processes, which is something we are working on,” says Abrikosov.
Text Monica Kleja
Translation Maxwell Arding
Photo Magnus Bergström