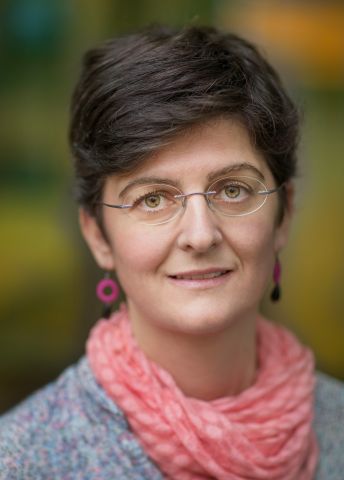Fuel cells filled with water work well – but only until the water has evaporated. Wallenberg Academy Fellow Anna Martinelli is seeking new materials for less costly and more heat-resistant fuel cells.
Anna Martinelli
Associate Professor of Materials Science
Wallenberg Academy Fellow 2016
Institution:
Chalmers University of Technology
Research field:
New ion and proton-conducting materials based on ionic liquids
In the Department of Applied Chemistry at Chalmers University of Technology Martinelli’s work is at once very concrete and fairly abstract. Essentially, she is engaged in exploring and experimenting with the movement of protons in fluid-like materials, but ultimately the results may be more tangible: more durable and more efficient fuel cells.
When Martinelli was growing up in Italy, her Swedish mother brought chemistry and engineering sets home for the children, along with artists’ materials. Anna and her siblings did finger painting and carried out experiments in the basement.
“My mother was a trained teacher and had very sound methodology. She let us explore shapes and colors, use tools and experiment with fabrics. Everything we did was based on the renowned Montessori Method. I think that may have had a hand in the research field I finally chose. Doing what I do – it helps to be handy!”
“Becoming a Wallenberg Academy Fellow has given me a unique opportunity to expand my research team and develop my ideas. I will also become part of a broader network. And a multi-year grant provides continuity. I can focus, and know that funding is secure for five years.”
Heat-resistant materials needed
Fuel cells are an electricity source of the future, beyond the era of oil and other fossil fuels. Electricity is produced in a fuel cell by an electrochemical reaction. The cell contains an anode (+) and a cathode (-), separated from each other. Hydrogen and oxygen are fed into the cell. When the hydrogen meets the anode a reaction occurs that splits the hydrogen protons from their electrons. The positively charged protons pass through a membrane in the cell towards the cathode, while the electrons move away via an outer circuit, i.e. an electric current is generated there.
When electrons in the circuit reach the other side of the fuel cell, they combine with the protons and with the oxygen in a new reaction. The only products of the reaction are water and heat. It is clean – but not efficient or cheap enough for large-scale use.
“The material currently used as a membrane is often a polymer, which, when filled with water, is a good conductor for the protons. But when the temperature rises above 80 degrees Celsius, the water evaporates and the system stops working, since the dry polymer cannot conduct protons. So we need to find new materials for use in fuel cells that can cope with temperatures over 120 degrees Celsius,” Martinelli explains.
She and her colleagues are working on alternatives to both the membrane material itself, and to the water it contains.
Molten salts or ionic liquids replacing water
One potential substitute for water they are testing is something called ionic liquids. These are compounds that are salts in the chemical sense, but that are liquid at room temperature. Just like water, ionic liquids are good at conducting both ions and protons. But unlike water, ionic liquids do not evaporate, and their conductivity actually improves at higher temperatures.
It would in fact be possible to continue to use the polymer as a membrane material in combination with ionic liquids. But it is expensive, so Martinelli wants to find an alternative to this as well. She is experimenting with porous silicon dioxide, with nanoscale pores, whose shape and size can be tailored.
“It’s not certain that the material is the best solution. But it will serve as a model system – a material where we can study the optimum shape and chemistry of the pores for retention of the ionic liquid, enabling it to act as a good conductor of protons and ions,” Martinelli explains.
One problem is that ions can bond to the walls of the pores, thereby becoming immobile. It might be possible to design the material to prevent this from happening. Or perhaps something can be made to happen that would be at least as desirable: getting protons to “jump” their way through the material, creating a kind of chemical domino effect. But this is hard to achieve.
“We will develop this by varying both the ionic liquid and the membrane. If we succeed in resolving all the problems involved, we will have found new materials offering potential for use in a fuel cell,” Martinelli adds.
New data the highpoint
She and her team are studying the atomic behavior of materials using several techniques, including vibration spectroscopy and nuclear magnetic resonance (NMR).
For her part, Martinelli likes it best of all when her research delivers new data, from which she can identify new patterns in graphs and diagrams.
“That’s the highpoint of my work. I have a strong visionary streak, and I’ve often mulled over ideas for a long time before I see the results. Once I have the results, I sometimes lose my drive. In my inner world I’m done, but then comes the phase of writing articles and persuading scientific editors that I’ve found something important. All this requires energy. For this reason I try to write while a project is going on, so I can get it down while it still feels fresh.”
Text Lisa Kirsebom
Translation Maxwell Arding
Photo Magnus Bergström