Projektanslag 2011
The artificial leaf: light-driven
Principal investigator:
Johannes Messinger, professor of biological chemistry
Co-investigators:
Göran Samuelsson
Jyri-Pekka Mikkola
Ludvig Edman
Mattias Marklund
Bertil Eliasson
Institution:
Umeå University
Grant in SEK:
40,3 million over five years
The greatest source of energy that exists is the sun. The sunlight that reaches the earth in one hour contains more energy than we humans consume in a year. The question is merely how to capture, convert, and store solar energy in order to exploit it optimally.
“The primary aim is to develop a more efficient method for transforming solar energy into a storable fuel, which can be used when needed to, for example, power vehicles or heat homes. Our goal is to demonstrate that this concept works efficiently al lab scale; subsequently others can work out the actual applications,” says Johannes Messinger, professor of biological chemistry at Umeå University.
Simple idea with certain challenges
Johannes Messinger’s research team is developing a membrane that, when placed in water and exposed to sunlight, can split water, producing hydrogen and oxygen. This work is possible due to funding by the Knut and Alice Wallenberg Foundation.
Briefly, the method involves absorption of sunlight by pigments or semiconductors, which then create positive and negative charges. These charges must then be separated so that the positive ones are gathered on one side and the negative ones on the other side of the membrane. When the positive and negative charges hit catalysts on the membrane surface, chemical reactions take place that lead to water splitting, yielding molecular hydrogen and oxygen.
“The idea is simple, but it entails a number of challenges that we need to solve, such as how we can channel the negative and positive charges to each side of the membrane.”
Another challenge is that a strong oxidizing power is needed to split the water, which means that there is the risk that the membrane itself might become oxidized and damaged. Other processes can also damage the membrane. Plants and cyanobacteria solve the problem by recreating the parts that are destroyed once every half hour, in full sunlight, but an artificial leaf has to be stable over a long period. What’s more, the technology needs to be inexpensive and efficient if it’s to be useful.
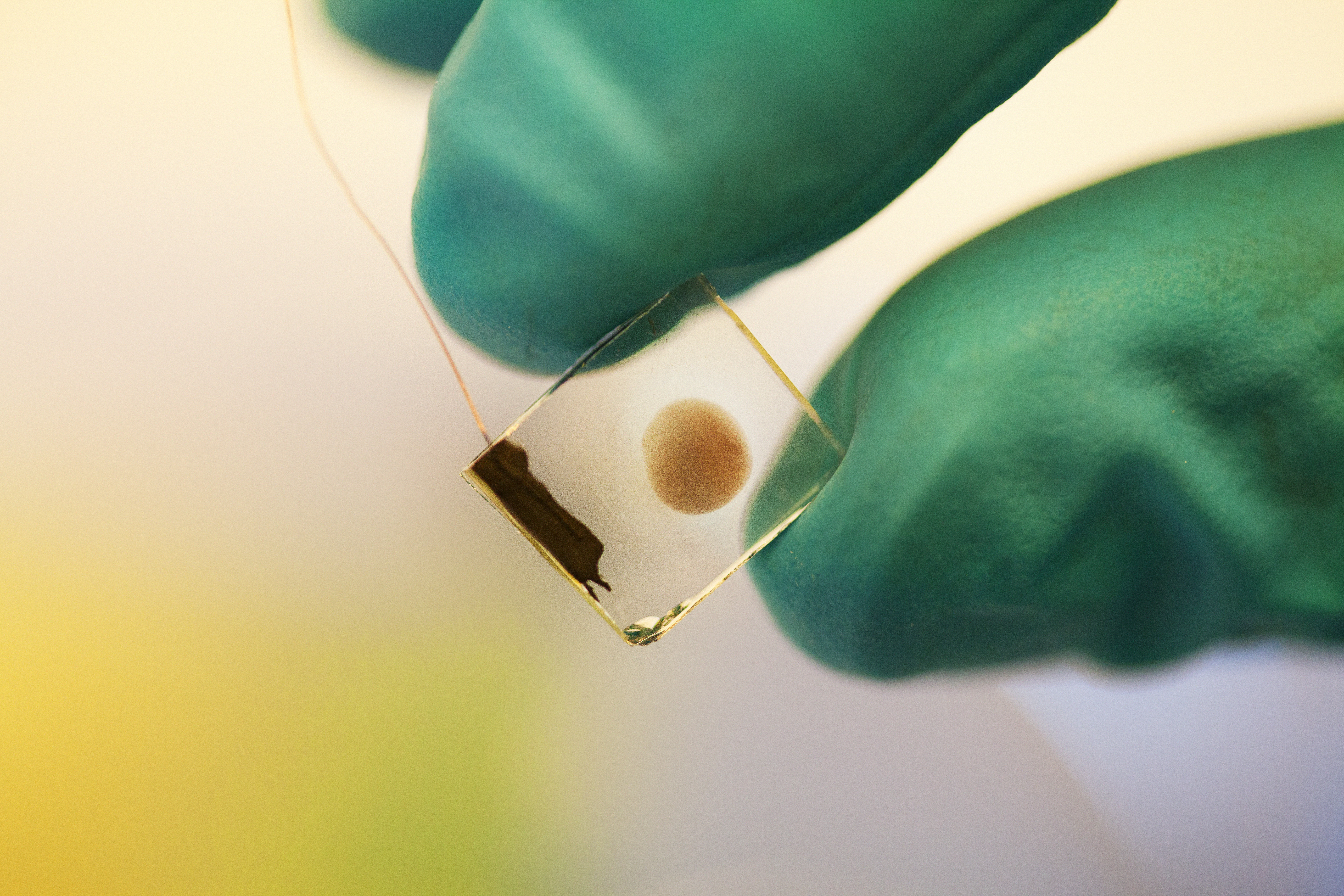
The secret lies in photosystem II
Throughout his research career, Johannes Messinger has been involved in studying natural photosynthesis and how water is split into oxygen and hydrogen-like products.
Splitting water is a process that takes place in green plants, algae, and cyanobacteria within a large protein complex known as photosystem II. Here water is divided into molecular oxygen, protons, and electrons. In artificial photosynthesis, the idea is to efficiently combine the protons and electrons to produce molecular hydrogen.
The scientists are trying to mimic natural processes with various types of catalysts. In order to split water, a metal-oxide complex is used, similar to what is found in photosystem II. And to combine protons and electrons into hydrogen, an active iron complex is used, that is similar to that found in enzymes – hydrogenases - of various microorganisms.
“The metal complex has to be stable, efficient, and inexpensive, and the metal must be available in large quantities. Manganese, cobalt, and nickel are some of the metals we use in our experiments.”
“The best thing about the process is that it’s entirely environmentally friendly. It starts with water, which is split and then returns to water in a cycle when the hydrogen is combusted, and its stored energy is used as heat or electricity.”
Better than the original
The researchers’ goal is to be able to create artificial photosynthesis that is more efficient than the original.
“Plants use energy from photosynthesis also to grow and multiply. For photosynthesis to function, the sunlight can’t be too intense. We want to be able to expose the membrane to intense sunlight. And of course the artificial system also lacks the growth phases,” says Johannes Messinger.
Ordinary plants reach barely one-percent efficiency in their conversion of sunlight into fuel. Johannes and his team are aiming, in collaboration with several other groups at Umeå University, to attain up to 10–15-percent efficiency.
“A research group at MIT in Boston is working with a concept similar to ours, except they’re using other materials. They’ve reached up to 2.5-percent efficiency, which shows that the idea works. The drawback with their process is that it’s too expensive to be mass-produced.”

Should be printable
To make the process more inexpensive to produce, Johannes and his associates are trying to make the membrane printable.
“The idea is to use roll-to-roll technology, similar to the way a newspaper is printed. That technology is already in use today in the production of the next generation of lighting, for example. But it will take several years for us to get that far.”
He also believes that once the technology of a membrane works, which powered by sunlight produces hydrogen from water, it will also be possible to transfer the method to construct devices producing methanol or ethanol from sunlight, water and CO2.
Johannes Messinger came to Umeå University in 2008 from the Max Planck Institute in Mühlheim an der Ruhr, Germany, where he was a group leader. In Umeå he is coordinating a team of researchers consisting of chemists, physicists, and plant biologists that have joined forces to contribute an environmentally friendly solution to the world’s energy needs.
“There’s truly an excellent scientific environment here at the Chemical Biological Center in Umeå. It’s just the place to advance this work on artificial photosynthesis.”
Text Carina Dahlberg
Translation Donald S. MacQueen
Photo Magnus Bergström