Project Grant 2020
Vascular organotypicity in health and disease
Principal investigator:
Professor Lena Claesson-Welsh
Co-investigators:
Uppsala University
Michael Simons
Christer Betsholtz
Elisabetta Dejana
Taija Mäkinen
Institution:
Uppsala University
Grant in SEK:
SEK 38,000,000 over five years
The primary function of blood vessels is to supply all tissues in the body with oxygenated, energy-rich blood, whereas the lymphatic system’s job is to collect leakage from the vessels. Blood and lymph vessels work very differently, depending on their location in the body. This variation is well known, but at present there is a lack of detailed knowledge about the unique structure of blood vessel cells, and the part they play in organ function. In particular, there is an absence of knowledge capable of linking the unique properties of the vessels to various diseases.
Linking function to disease
A project funded by Knut and Alice Wallenberg Foundation aims to map blood and lymph vessels in three of the body’s organs: the central nervous system, the skin and the aorta.
“We intend to create an atlas that links information about blood vessel cell expression patterns of genes and proteins to function, and eventually also to various diseases. We want to understand how the characteristics of organotypic blood vessel cells cause common diseases to affect a given organ,” says Lena Claesson-Welsh, a professor at Uppsala University.
The project is centered on these three organs because the characteristics of the blood vessel cells in them differ from each other to the greatest extent. The central nervous system comprises the brain and the spinal cord, and is the most energy-intensive organ in the body. This means it requires a highly intricate network of blood vessels to reach the billions of neurons. Meanwhile, the brain is protected by the ingenious blood-brain barrier, which effectively prevents fluids and molecules leaking out of the vessels.
Properties of skin blood vessel cells include temperature regulation, and the cells can open and leak. The aorta is the largest artery in the body, and is capable of withstanding the large pressure changes that occur as the heart pumps out blood.
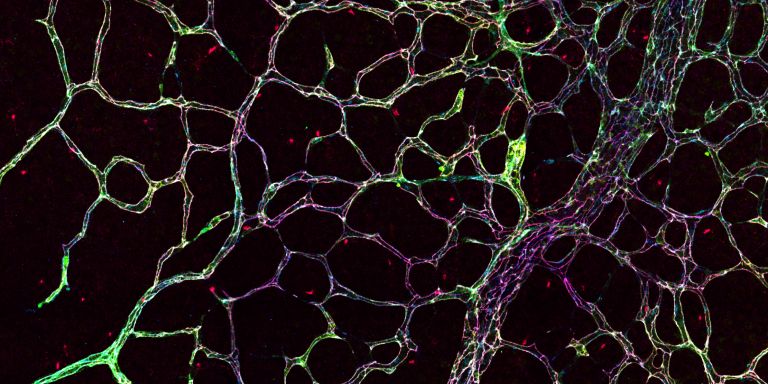
The various properties of blood vessel cells are regulated by the genes and proteins that are expressed in the individual cells. The aim of the project is to draw a map showing precisely which genes and proteins are active in each individual cell type. The map can be used to learn more about the vessels’ organotypic functions, and will include both the internal endothelial cells, and the outer support cells.
The researchers hope to use the map as a basis for answering questions about the structure of the blood-brain barrier, how the aorta copes with pressure changes, and other issues. The team also wants to link the conclusions to the diseases affecting the vessels. Some diseases originate from specific genetic mutations, but although the mutations are present in all blood vessel cells, they only cause disease in certain organs.
“We wonder what happens in organotypic blood vessel cells to cause certain organs to become diseased, for instance why mutations present in all blood vessel cells only lead to deformed blood vessels in the skin. To find out we’ll have to establish the functions of individual cells,” Claesson-Welsh says.
Mapping genes and proteins
One method the researchers are relying on is single-cell RNA sequencing. This enables them to open the door to individual cells in the blood vessels to see which mRNA molecules are active. This information is supplemented by determining which proteins are present in each cell.
“Traditionally, you study one protein at a time, but it’s important for us to examine several simultaneously to see how they interact. We’ll need to develop new methods so we can really distinguish between them.”
The project has gathered some of the world’s foremost researchers in the field of vascular biology at the Rudbeck Laboratory in Uppsala. The team also has access to clinical material from the U.S., Finland and Sweden.
“We’re one of the few research centers in the world conducting blood vessel research at this level. And we have brought together a unique team, possessing long experience and in-depth knowledge of both healthy and diseased tissue.”
Several of those behind the project are also renowned for their development of techniques and methods.
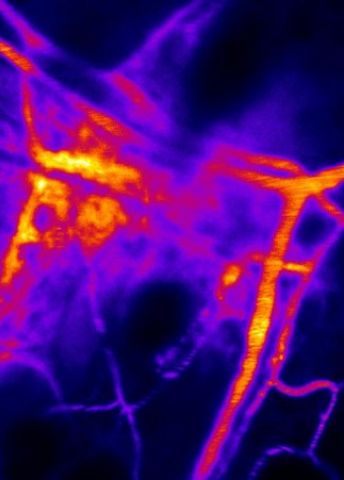
“We’ve already established a number of techniques for studying blood vessel cell function. Among other things, we can study leakage from blood vessels in living tissue with high resolution down to individual cells in the tissue.”
In situ study essential
One of the things that makes analyses more difficult is the low number of blood vessel cells in each organ. Only 1.5 percent of all cells in the skin are blood vessel cells. Moreover, blood vessel cells must be studied in situ in the organ, since the flow of blood itself forms part of the cells’ function.”
“It’s usually simpler to culture individual cells in a dish in order to learn more about their characteristics. But blood vessel cells are impacted by blood flow, so we have to analyze them in the intact tissue.”
The highly detailed blood vessel atlas that is intended to result from the five-year project may lead to higher precision therapies. Claesson-Welsh likens it to replacing a sledgehammer with a well-directed pinprick.
“Preventive treatments may also assume ever greater importance in the future. If we understand the origin of different diseases, we can also learn how to keep these cells healthy. We already know a fair amount, but we’ll be able to obtain more detailed information in the future.”
Text Magnus Trogen Pahlén
Translation Maxwell Arding
Photo Dirk Pacholsky, Dominic Love, Yi Jin, Yindi Ding