Project Grants 2012
Novel cancer targets within nucleotide metabolism
Principal investigator:
Thomas Helleday, Professor of Chemical Biology
Co-investigators:
Karolinska Institutet
Sören Lehmann
Eva Hellström Lindberg
Jonas Bergh
Ulrika Warpman Berglund
Stockholm University
Ingrid Granelli
Pål Stenmark
Uppsala University
Per Artursson
Institution:
Karolinska Institutet
Grant in SEK:
SEK 35,5 million over five years
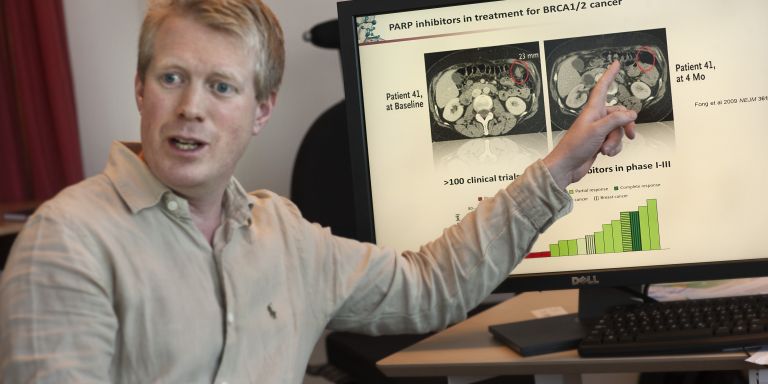
A few years ago, Thomas Helleday, Söderberg Professor of Translational Medicine and Chemical Biology at Karolinska Institutet, tested an exciting new treatment against hereditary breast cancer, now tested in clinical phase III studies world-wide. It initially looked very hopeful. The tumors shrunk and vanished. But suddenly, the cancer cells changed. They found a way around the treatment and began growing again. The patients got to live a few years extra, but the treatment did not cure the disease.
This is a common story in cancer research. Hope turns into realism that targeting the genetics of cancer has limitations. Now, Thomas Helleday, who is the coordinator of a project financed by the Knut and Alice Wallenberg Foundation, believes that it is time to rethink things when it comes to cancer. Researchers have long tried to develop targeted treatment fighting the cancer genetics. But the limitations of the PARP inhibitors has taught him that tumors consist of such a motley crew of cells that targeting a single genetic change maybe only present in a subset of the cancer is not enough.
“Cancer differs a great deal between different patients. But there are also a lot of differences between different cancer cells in a single tumor. In targeted treatments, we only kill a part of the tumor,” says Thomas Helleday and continues:
“Now, we have to begin thinking about what all cancer cells have in common and what distinguishes them from normal cells.”
Cancer cells contain a lot of oxygen radicals
One thing that is common to cancer cells is the very fact that they easily change their genes, mutate, and develop resistance to treatments. The ability to mutate is mainly caused by cancer cells forming a large amount of oxygen radicals, reactive molecules that change the cell. A normal cell wouldn’t live for long when it is filled by oxygen radicals. But for a cancer cell, this increase in ability to mutate is an advantage.
At the same time, the radicals may not cause too much damage. If the genetic changes get to large, cancer cells will also die.
A few years ago, Thomas Helleday began thinking about if it would be possible to upset this balance; if the scales would tip in the right direction if the radicals were given greater leeway in the cancer cells. The research team therefore began to investigate what happened if they disabled genes that protect against oxygen radicals. The experiments were done in colon cancer cells and the researchers tested turning off hundreds of different genes. His ideas proved to be correct. In some cases, the cancer cells were fully paralyzed.
“We had some ten real hits,” says Thomas Helleday.
One of these captured his interest; a gene that contains the code for a special enzyme, a so-called nucleotide hydrolase, which protects cancer cells from getting overly damaged genetic material.
Protects cancer cells from building defects into the genetic material
The body's genetic material is built of so-called nucleotides. Nucleotide hydrolases take care of nucleotides that have been destroyed by oxygen radicals so that they are not built into the genetic material. When Thomas Helleday read about the enzyme in the research literature, he realized that it could the Achilles heel he was looking for:
“The enzyme was discovered in bacteria. When the enzyme was removed, the mutation frequency in the bacteria increased about 10,000 fold. They mutated as much as possible,” says Thomas Helleday.
So it is an important gene in bacteria. But when other researchers disabled the same gene in mice, nothing happened at all. The mice managed fine without the gene.
What made Thomas Helleday raise his eyebrows was an experiment where researchers, in an attempt to see an effect of nucleotide hydrolases in mice as well, disabled a combination of two different genes. They crossed mice that had a genetic mutation that caused them to easily get lung cancer, with the mice that lack nucleotide hydrolases. The results surprised the researchers:
“They thought that the mice would be struck by lung cancer even earlier in life. But instead, they got no lung cancer at all,” says Thomas Helleday.
The researchers thought the experiment was a failure. But for Thomas Helleday, a light went on.
“I interpreted this to mean that lung cancer cells need nucleotide hydrolases to survive. When they removed the enzyme, all the other cells survived, but not the cancer cells,” he says.
The goal is a molecule for clinical trials
His team set out and worked for many years and proved the hypothesis to be right. The potent inhibitors the team developed stop cancer cell growth in both cell cultures and in mice models. Now, Thomas Helleday's goal in the course of two years is to develop a substance that is also stable in the human body and can be tested in clinical trials. For this project, Thomas Helleday and his colleagues have received SEK 14.2 million from the Knut and Alice Wallenberg Foundation. Among his colleagues in the project are a few of Sweden's brightest medicine developers. Together, Thomas Helleday believes that they will be successful:
“I'm convinced of it,” he says and smiles.
If the researchers succeed in developing a molecule fit for clinical trials, the Knut and Alice Wallenberg Foundation will finance the project for another three years.
Text Ann Fernholm
Translation Semantix
Photo Magnus Bergström