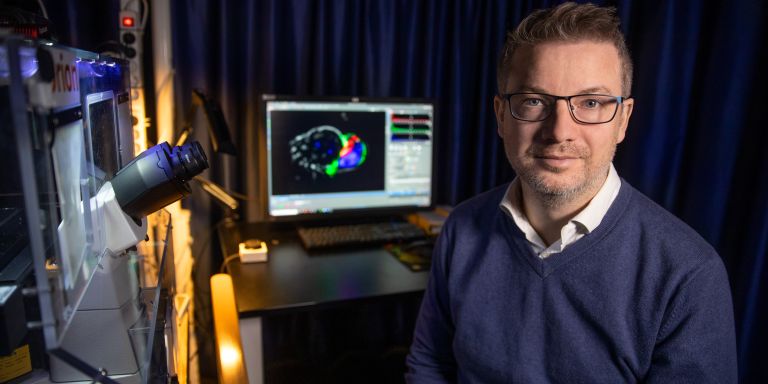
Life-threatening infections, hyperinflammation and cancer may result from mutations in DNA that impact the immune system. Yenan Bryceson at Karolinska Institutet is studying defective immune cells, and seeking explanations in proteins, genes – and in the large area of DNA that contains no genes.
Yenan Bryceson
Professor of Translational Immunology
Wallenberg Academy Fellow, prolongation grant 2019
Institution:
Karolinska Institutet
Research field:
Genetic mutations in cytotoxic T cells and natural killer cells
Many diseases are influenced by our genes, often by several of them acting in concert. But there are also monogenic conditions, i.e. those due to mutations in a single gene. The research that Bryceson is conducting as a Wallenberg Academy Fellow is aimed primarily at diseases of this kind. He is studying two common immune cells: cytotoxic T cells and “natural killer cells.” Both protect the body by killing infected cells and abnormal cells, such as cancer cells. If these immune cells are seriously defective, this becomes evident immediately after birth, when the infant may suffer “hyperinflammation.” But clear pathological effects of variants in individual genes can sometimes also be found in adults. Minor defects that are not noticeable early in life can manifest themselves later on in the form of cancer, infections or even neurological problems.
“We’re not really sure yet what causes the neurological symptoms. It may be that these cell types have a key role in fighting viruses in the central nervous system,” Bryceson says.
“As a Wallenberg Academy Fellow, I get to meet researchers from other environments. We exchange ideas and experience of issues that everyone is wrestling with, such as how to create a good project environment for graduate students.”
Discovery followed by years of research
Bryceson’s research team often bases its work on patient cases, where one or more members of a family experience serious symptoms. Their entire DNA is sequenced in an effort to find deviations, and blood and tissue samples are taken to study the activity of individual cells. Since the task of genes is to code for the proteins that the cell produces, genetic defects means that too much or too little of a protein is produced, or it is produced in the wrong form. Bryceson is studying in detail which proteins are synthesized by the immune cells, how they interact with other proteins, what processes they are involved in, and how a mutation can destroy them.
“The more we understand how individual proteins affect the cell and immune system, the more we will understand the disease and what can be done to treat patients,” Bryceson explains.
Sometimes he finds new changes in known disease genes; other times he discovers something quite unknown. A couple of years ago the research team discovered a mutation in an unknown gene that turned out to increase both susceptibility to virus infections, and the risk of developing blood cancer. He thinks these discoveries are the most interesting of all:
“In these cases samples from a single family can mark the start of a large-scale multi-year research project.”
Searching for explanations in the space between genes
A mutation has been found in fewer than 40 percent of all the patients that researchers believe are monogenic. The cause of most of these diseases therefore remains unknown, and Bryceson believes the explanation may lie in the regions of DNA that are not genes. This is because genes only account for between one and two percent of DNA. Large parts of DNA have no known function, but we know they contain sequences that control which genes are active.
“We’re trying to develop methods to determine when genes in immune cells are not active in the right way, and to identify the places in the non-protein-coding genome that cause these effects.”
Finding standardized ways of searching for mutations in non-coding DNA is a major challenge Bryceson faces in his future research. To date, he and his team have developed a number of genetic tests that are used by other researchers, as well as tests that can be used in diagnosing patients. He hopes they will start to be used in the medical sector over the next few years.
“If a genetic diagnosis is made even before birth, or immediately after it, treatment can begin before serious complications arise,” Bryceson says.
But treatment is no simple matter. It often entails a bone marrow transplant, enabling the child to develop a completely new immune system. But better options may become available in the not-too-distant future. New gene editing techniques like CRISPR/Cas9 offer the potential to modify the DNA of the patient’s own stem cells, inducing them to produce immune cells without the harmful mutation.
Bryceson’s mother is from the U.S., his father from Tanzania. Both are researchers, one in marine biology, the other in anthropology, so the idea of a career in research came to him early. He was born in East Africa, but his parents moved to Norway when he was just six years old, and he grew up there. Studying internationally did not feel like a big step, so he moved to the U.S. and then Sweden. He has always been fascinated by how nature and life work, and when he took courses in biochemistry, he discovered that the most minute, molecular cycles inside the cell were enormously interesting.
“Pure research was originally my thing, but it’s a huge privilege to be able to work so close to health care itself. I get to see how insights from my scientific research are of vital importance to patients as well. It’s extremely stimulating.”
Text Lisa Kirsebom
Translation Maxwell Arding
Photo Magnus Bergström