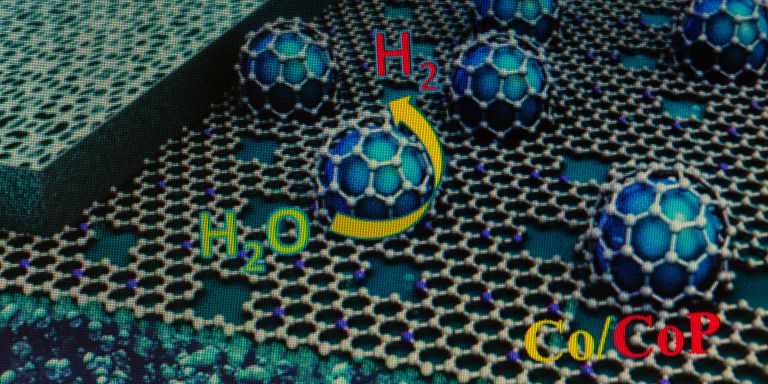
New high-efficient electrodes made from porous carbon membrane may have important applications in the environment and energy sectors. Jiayin Yuan is tailoring carbon electrodes that can be used to convert carbon dioxide into chemicals.
Jiayin Yuan
Professor in Materials Chemistry
Wallenberg Academy Fellow prolongation grant 2022
Institution:
Stockholm University
Research field:
Nanoporous carbon membranes for environmental and energy applications
Jiayin Yuan shows his new lab, which looks out over a leafy courtyard. Here he is developing small porous carbon membranes that may be of great future benefit.
“Due to the climate changes we must commit to sustainable development. Many research fields are involved. In our case, it’s about developing a new generation of electrocatalysts. They are based on unique porous carbon structures.”
Porous active carbon has long been used in water treatment and medicine, among other things. The porous carbon membranes on which Yuan is researching are macroscopic, made up of closely interconnected nanoscale pores. The total surface area of the pores in the material, even a small piece, is huge – about the size of a football pitch, he says. Applications for nanoporous carbon membranes include electrodes, electrical conductors, and electrodialysis.
“They are non-metallic, so they don’t involve any risk of hazardous metals leaking out, and they are much cheaper to manufacture on a large scale.”
Macroscopic carbon membrane
Design is critical for a carbon membrane to be sufficiently efficient, Yuan explains. The type he is refining in his new lab is called “porous hetero-doped gradient carbon membrane”. It has two major advantages:
“One is the membrane form, which means that we don’t need any binder to hold the carbon electrode together. The other advantage is that the structure provides an excellent platform for various functions. For instance, we can achieve very high conductivity.”
Yuan and his fellow researchers have published a number of scientific articles in which they have demonstrated that this nanoporous carbon membrane electrode is much more efficient at producing and converting energy than traditional carbon powder electrodes.
“One example is about reducing CO2, where we electrochemically take care of carbon dioxide and convert into something useful.”
Optimizing the design
Yuan explains how he was attracted to research early on, having completed a BSc in chemical engineering in China, his home country:
“It’s my dream job. I like research, but also how the work is organized. You can’t do everything in your own lab – that’s basically impossible these days. We need to work together and exchange knowledge with others, and have a broad international platform.”
He moved to Stockholm from New York State and Clarkson University. Before then he had lived in Germany, where he received his PhD, began his career as a researcher, and became team leader at the Max Planck Institute of Colloids and Interfaces in Potsdam.
At Stockholm University Yuan’s goal is to put together his research team, and understand the fundamental mechanisms driving the electrochemical operation in the porous carbon electrodes.
“This kind of funding is hugely important for young researchers, and for basic research. The grant will enable me to set long-term research goals.”
“We want to produce as high-efficient electrodes as possible for a few electrochemical processes. Carbon dioxide reduction is one of them. We also work on an ion separation process that is just as important. A membrane that can exploit a salt gradient in water, for example where river water meets sea water, can be used to generate energy.”
Yuan is conducting his research as a Wallenberg Academy Fellow, and hopes that by the time the project is over, in five years’ time, his team will have achieved a high enough level of carbon dioxide reduction to make the technology commercially attractive.
“What’s so cool is that it can be combined with energy from renewable sources. Wind power is hard to store. But we can combine the technologies to produce fluid forms of energy that can be transported and used at any time. The turbine converts wind into electricity – the electrochemical process uses the electricity to convert carbon dioxide into formic acid or methanol, for example.”
Unified whole at a reasonable cost
The efficiency of the carbon membrane is enhanced by giving the pores more precise structures and functions with the help of heteroatoms, such as nitrogen, sulfur and boron.
“Most of the materials we are using are synthesized in this laboratory. We mainly use raw materials from chemical companies, but we plan to study biomass as a starting material in the future.”
Carbon dioxide reduction is tested and analyzed in various electrochemical experiments carried out in the lab. Yuan spends hours in front of his computer in search of the optimal process.
“The challenge is to unite the factors we know about to form a coherent whole. It’s one thing to check a single parameter, but it’s really hard to check several at the same time. It’s an extremely complex system, and we have to compromise in various ways all the time.”
Another key issue is the cost of materials.
“Once we understand the fundamental mechanisms, we will approach the application phase. But if the raw materials are too costly, the technology will not be able to create real change in the society. The dream, of course, is that the porous carbon membrane electrodes we make will one day be available on the market – that they will be instrumental in energy-efficient and environmentally friendly solutions.”
Text Susanne Rosén
Translation Maxwell Arding
Photo Magnus Bergström