Project Grant 2015
Characterization of New Superheavy Elements (Lundium)
Principal investigator:
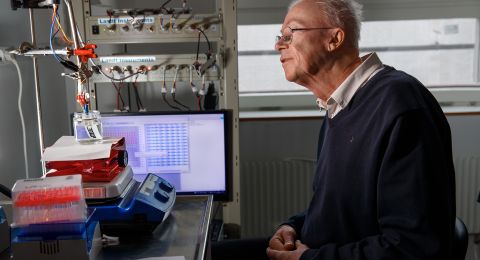
Dirk Rudolph, Professor of Nuclear Physics
Co-investigators:
Sven Åberg
Gillis Carlsson
Universität Mainz
Christoph E. Düllmann
Institution:
Lund University
Grant in SEK:
SEK 38.3 million over five years
Elements with more than 103 protons in their nucleus are called “superheavy”. A handful of these elements have been known for decades, but four new members joined the group in December 2015, when the International Union of Pure and Applied Chemistry (IUPAC) acknowledged elements 113, 115, 117 and 118. All of them are artificial, and highly unstable. Complicated measurements are needed to find and record the particles before they disappear. Researchers in Lund provided key parts of the evidence for the existence of element 115.
With help of a grant from the Knut and Alice Wallenberg Foundation, the research team in Lund will now be developing instruments and methods to improve measurement of the new elements, and perhaps ultimately create new, even heavier ones. This will require a combination of advanced experiments and theoretical computations. In Lund there are researchers in both fields.
“Sometimes we ourselves don’t really know what we are observing in our experiments. When this happens we turn to our colleagues in the Department of Mathematical Physics one floor up, and discuss the results,” says Dirk Rudolph, Professor of Nuclear Physics.
The close relationship between theory and experiment was one of the reasons he moved from Germany to Lund University in the 1990’s. His colleague, Sven Åberg, Professor of Mathematical Physics, agrees that close collaboration is essential.
“We don’t just make theoretical computations on our own – we engage in a dialogue with the experimentalists to discuss why they see the results they do in their experiments. And sometimes we are the ones who can suggest something they can try to measure. The dynamic between us is really important,” Professor Åberg explains.
Superheavy elements born out of collisions
Superheavy elements are created when the atomic nuclei of other elements are made to collide with each other so they fuse to form a new, larger nucleus. The new nucleus decays very rapidly – in fractions of a second. The radiation emitted when the atomic nucleus decays or changes in various ways is measured. This enables the researchers to draw conclusions about how large the particle was originally, and also about its internal structure.
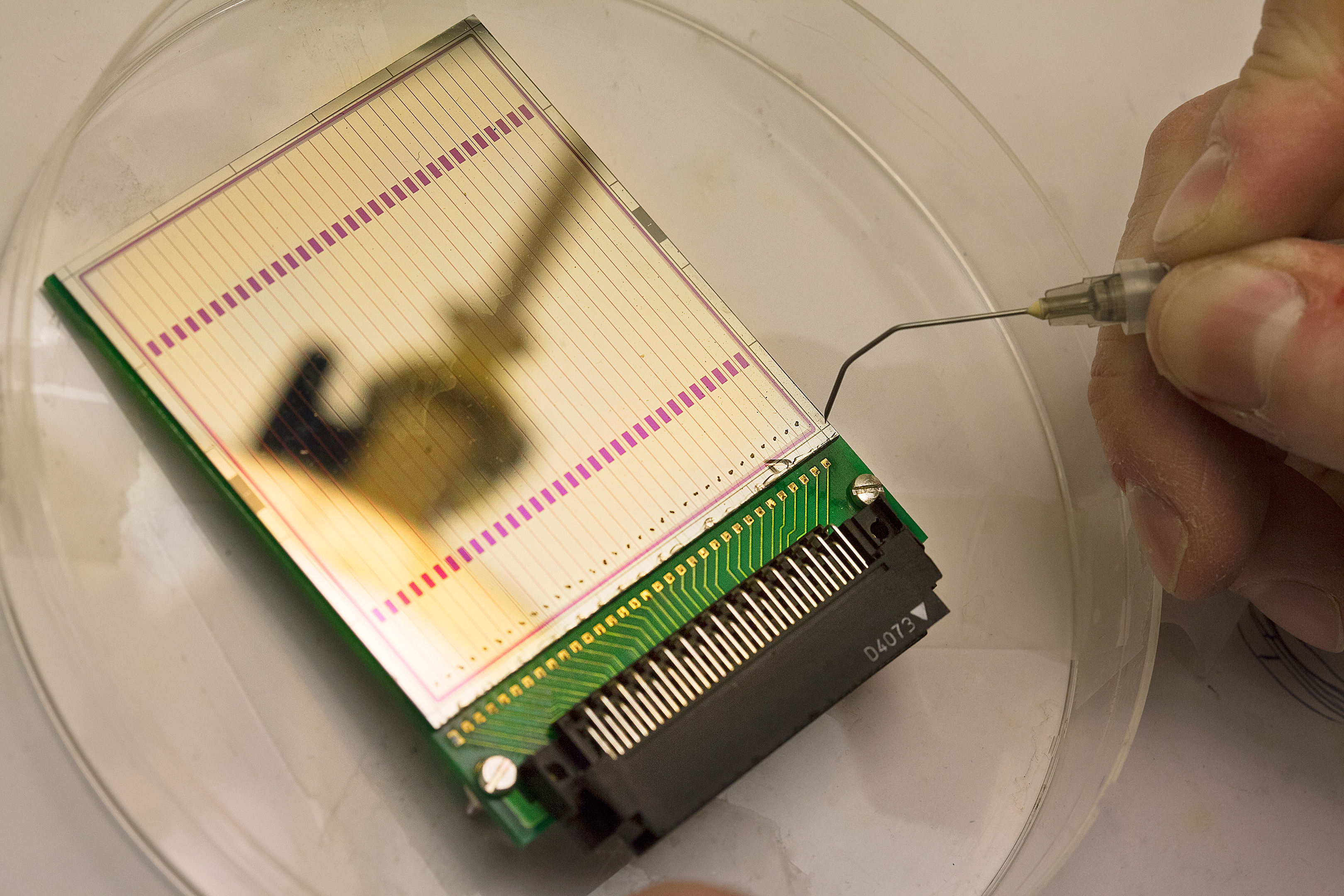
The Lund researchers conducted their experiments on element 115 at a research facility in Germany. A thin film of the element americium was bombarded with calcium ions. After six trillion collisions the researchers had managed to measure 30 decays that could be shown to have come from element-115 nuclei. This was compelling evidence for the existence of a new element.
The team in Lund will now be building an even better measuring instrument. This, together with a more powerful particle accelerator, will make study of superheavy elements ten times more efficient. This means that the same experiment can be carried out in one-tenth of the time – or the same amount of time can be spent, but will yield much more detailed information. The experiments are being performed in collaboration with researchers in Germany, using a particle accelerator in Darmstadt.
More neutrons would make the nuclei more stable
The researchers also hope to be able to produce and discover even heavier elements using the new instruments.
“These elements, which are created by collisions, have plenty of protons but relatively few neutrons. Our theoretical computations suggest that the nuclei would be much more stable if they had more neutrons. This is difficult to achieve experimentally. But maybe such elements can be found in the remains of meteorites,” Professor Åberg comments.
If it were possible to find or create superheavy elements that did not immediately decay, what could they be used for? It is impossible to say, according to the researchers. After all, it has not yet even been possible to determine their chemical characteristics.
“The first atoms of americium were synthesized in the 1940’s. Twenty years later the element began to be used in fire alarms, and still is. Who could have imagined that americium would be used to save human lives throughout the world?” adds Professor Rudolph.

“Lundium” – an element of the future
It is equally impossible to know whether the 119th element of the future will attract as much attention as 115 did. For a time, Professor Rudolph was more or less under siege from the Swedish and international media. He calls the period “stressful but instructive”.
“It was a kind of hype – something those involved in basic research are not used to. Also, the English language press release was issued too early, a few days before we were published in Physical Review Letters. Fortunately, they were glad of the attention. If it had been Nature or Science, they would have cancelled publication…”
Suggested names for element 115 are currently being considered. The Swedish research team has, somewhat jokingly, called it “Lundium”, but at the moment it is likely that the name will be chosen by a Russian-American research team. They were the first to create 115 particles, although the Swedish team came up with more persuasive evidence for their existence.
“We don’t get to choose the name this time. But when we find elements 119 and upwards….THEN we will go with Lundium,” Professor Åberg says, laughing.
Text Lisa Kirsebom
Translation Maxwell Arding
Photo Magnus Bergström