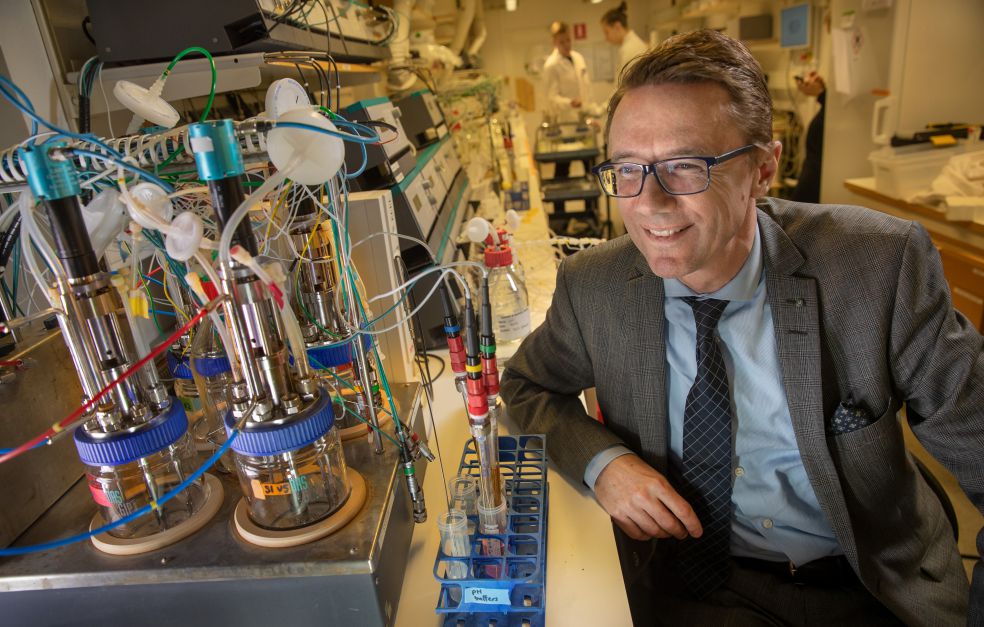
Yeast cells as both models and factories – this is what lies at the heart of Wallenberg Scholar Jens Nielsen’s research. He is studying genetic changes, and analyzing data to obtain new insights into the human body and major diseases. But he can also get yeast to produce green fuels and chemicals.
Jens Nielsen
Professor of System Biology
Wallenberg Scholar
Institution:
Chalmers University of Technology
Research field:
System biology and biotechnology. His research includes control pathways in eukaryotes to develop efficient cell factories for sustainable synthesis of biofuels and chemicals.
Yeast has long been a favorite organism among researchers, being easy to cultivate in the laboratory. The yeast genome has been mapped, and we are gaining a greater understanding of cell metabolism – the complicated process whereby genes cause the cell to synthesize substances in different quantities at different times. Cell metabolism is precisely what Jens Nielsen, Professor of System Biology at Chalmers University of Technology, is researching. He is using computer models to map and analyze yeast and human cells alike, sometimes using yeast to understand human biology.
“Metabolic changes occur in human cells when people get sick. Perhaps the most dramatic example is that of cancer cells, where a major transformation occurs,” Nielsen says.
His research team has made mathematical analyses of samples from the health care sector. This has enabled them to identify metabolic changes that are typical of kidney, bladder and prostate cancer, for example. They are now developing urine and blood tests that will enable them to study biomarkers, see how concentrations of given substances rise and fall. This will in turn make it possible to detect relapses, or see whether a therapy is having the desired effect. The biomarkers are now being tested at Sahlgrenska University Hospital and elsewhere.
Nielsen’s mathematical models also enable researchers to find new interrelationships in the body that are associated with complex conditions, such as various forms of cardiovascular disease.
“We have maybe five thousand metabolites floating around in our bloodstream, and many of them are closely related to each other in the sense, for example, that one can be converted into another via a specific enzyme. Our models and AI systems can see these links, so we can see that an increase in one substance coupled with a decrease in another is probably due to an enzyme altering its activity.”
“Being chosen as a Wallenberg Scholar has really made all the difference. Although I receive funding from other sources also, the Wallenberg grant has enabled me to commit to riskier projects – like the initial experiments on human biomarkers.”
Mini chemical factories
The software can also be used to examine new questions, and plan experiments on yeast cells. They can be used as miniature factories that have been genetically manipulated to produce the desired substances. The concept is not a new one; we have used yeast for thousands of years to break down sugar and make ethanol – alcohol – in the form of bread, beer and wine. But yeast can do much more.
“Many industrial chemicals are currently oil-based, and it would be better if they could be manufactured in a more sustainable way. We have managed to convert yeast metabolism into synthesis of nearly thirty chemical substances that yeast does not produce naturally,” Nielsen explains.
To make this happen the scientists have to insert foreign genes in the yeast. If yeast cells are exposed to electricity, their membranes open up a little, allowing new genes to be inserted. This is done using the Crispr/Cas9 gene editing technique. The yeast cells then grow in a solution, during which time they synthesize the substances for which the new genes code. After a while the yeast can be filtered off, leaving behind the desired substances in the solution.
One product that yeast ought to be able to make is high-energy density biofuel, known as biodiesel, used in airplanes and heavy-duty vehicles.
“We’ve tried to get the yeast to first produce fatty acids, and then to carry out a chemical process converting those acids into hydrocarbons. The fatty acid production was successful, but conversion into hydrocarbons is not efficient enough yet.”
A new path to biodiesel
Some years ago he was optimistic that the problem would soon be resolved, but then the price of oil dropped, and so did interest in biofuels. He now sees that demand is growing once more, this time because of social pressure from the climate movement. And he has begun to believe in a new path.
“We have recently seen that aviation companies and energy companies are buying increasing quantities of fatty acids, which they are then converting into hydrocarbons. If we make the process by which yeast synthesizes fatty acids more efficient, perhaps the chemicals industry can take charge of the last step itself, and produce biodiesel using our raw material.”
Fatty acids can also be used in numerous other applications, such as pharmaceuticals, foods, dietary supplements, cosmetics and chemicals. Nielsen and his colleagues have already registered a number of patents, and started a company to commercialize the technology. The team is also carrying out basic research to learn more about how the metabolism relates to other cell functions, and to develop the mathematical models for data analysis. But it is no coincidence that Nielsen, originally an engineer at DTU – the Technical University of Denmark, is devoting so much energy to research offering potential for new products.
“I’m not from an academic family. My relatives worked in manufacturing and trade – my father ran a factory. It’s always been important to me to find practical uses for what I do, and to contribute to change. I’m happy to work in partnership with industry, and try to figure out the solutions they need. They don’t want me to improve their processes – they’re really good at doing that themselves. They want me to identify the issues that will be important to them in five years’ time.”
Text Lisa Kirsebom
Translation Maxwell Arding
Photo Magnus Bergström