Project Grant 2022
The routes of glioblastoma and their patient-specific vulnerabilities
Principal investigator:
Sven Nelander, Professor of Integrative Cancer Research
Co-investigators:
Uppsala University
Lene Uhrbom
Katarzyna (Kaska) Koltowska
University of Gothenburg
Rebecka Jörnsten
Stockholm University
Mats Nilsson
Institution:
Uppsala University
Grant in SEK:
SEK 38,200,000 over five years
Professor Sven Nelander is heading a project involving five research teams from several Swedish universities. Together they have created a new strategy for mapping the growth of glioblastoma – an incurable and highly aggressive brain tumor. Greater understanding of how the tumor cells invade the brain may lead to better ways of treating them with therapeutics.
“If we can identify the genes that control the invasion of the tumor cells, we hope also to be able to develop ways to prevent the tumor’s invasion. The aim is to tailor individual therapies for each patient in the form of existing treatment methods as well as new ones,” says Nelander, who is Professor of Integrative Cancer Research at Uppsala University.
At present, glioblastoma is treated by surgery followed by radio- and chemotherapy. Yet even then the patient may survive for less than a year.
Tumor cell biobank
The platform for the project is a biobank called Human Glioblastoma Cell Culture (HGCC) in Uppsala. The biobank consists of cells cultured from tumor cells removed from patients. The researchers can use these cultured cells to recreate the tumors in various animal models. Ways have already been developed to identify and recreate various forms of tumor cells.
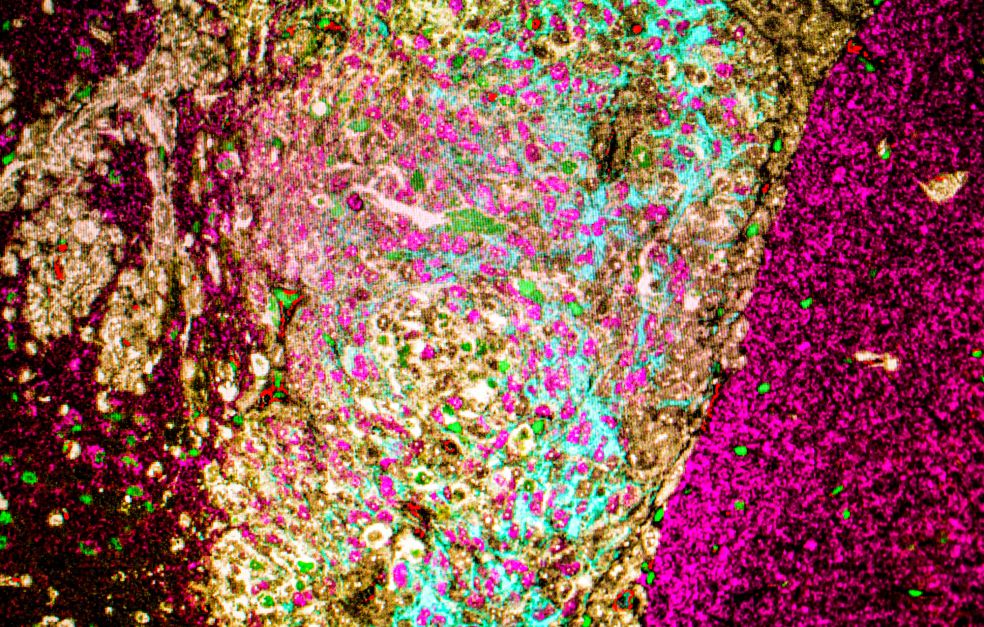
“If you examine a brain tumor under a traditional microscope, you will see that the cells differ, and that they interact with normal brain cells in different ways. Some climb on blood vessels; others pierce right through tissue or are led by neural pathways. We’ve found ways to recreate most of these kinds of tumor cell in the lab,” Nelander explains.
The researchers are examining growing tumors to identify correlations between different tumor cells and the way they invade the brain. Mats Nilsson’s research team has developed its own method for in situ-sequencing for this purpose. The method enables the researchers to ascertain which genes are expressed in each individual cell in the tumor tissue itself. Once they have identified the group of key genes that control the invasion, that knowledge can be used to develop new therapies.
Creative use of gene editing
To identify the key genes the researchers are developing a new model based on the CRISPR/Cas9 gene editing tool. They are combining use of the tool with large-scale data analyses so as to identify the genes behind the aggressive tumor growth.
“We’ve found a really creative way of using the gene editing tool to inactivate selected genes on a very large scale and also of measuring the effect of inactivation. It’s very effective and gives us access to a huge amount of data.”
Simply put, a large number of gene editors are created, each with a different target in the DNA of the tumor cells.
After selected genes have been edited out, the effect of this on the living cell is analyzed. The analysis is based on enormous quantities of data and requires access to the very latest computational methods.
The last stage of the project is to use the knowledge acquired to create new treatment methods, both in the lab and in animal models.
“That will also enable us to evaluate our methods alongside existing therapies to see how they can be combined. The evaluation will then form the basis for clinical trials of therapies tailored to each patient.”
Modern analytical methods
The key to the strategy behind the project is the combination of experiments using animal models, large-scale genetic computational methods and modern calculation methods such as machine learning.
“To some extent the aim of the project is to replace fairly unsuccessful existing models with a combination of large-scale genetics and computational methods. We’re at the forefront of a wave of similar data-driven initiatives in medical and health care research,” Nelander says.
Throughout his research career Nelander has sought to combine mathematical models with various forms of experimental research. A move to Uppsala University appealed to him partly because of the wealth of patient material gathered there in various biobanks. His research team has been involved in going through the material systematically with the help of modern computational methods.
He stresses that ready access to patient material is essential to the project’s success. For that reason it is also important to share both methods and findings with other research teams. He hopes the knowledge accumulated about the HGCC biobank will be a source of success for others.
“Our project will make an important contribution to the biobank itself. It will now be possible to improve the ongoing collection of cancer cells as well as the prospects of culturing them. And it’s essential that we share our findings, models and data with researchers all over the world, since we’re all trying to achieve the same goals,” Nelander explains.
Text Magnus Trogen Pahlén
Translation Maxwell Arding
Photo Magnus Bergström