Christoph Langhammer is exploring boundaries. But they are not external boundaries; they are internal ones – inside the nanoparticles he is building to create high-speed ultra-sensitive hydrogen gas sensors. The materials behave differently at the boundaries. He wants to exploit these traits to improve the particles.
Christoph Langhammer
Professor of Chemical Physics
Wallenberg Academy Fellow 2016
Institution:
Chalmers University of Technology
Research field:
Nanotechnology, materials science, nanoplasmonics
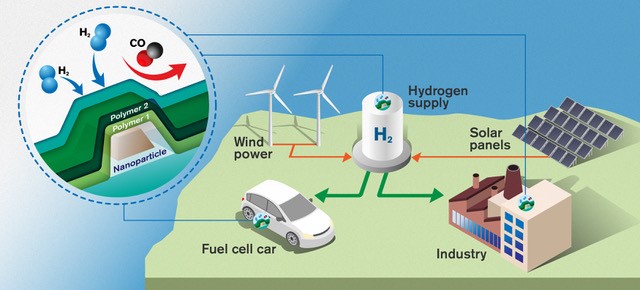
As society is moving away from fossil-based energy, hydrogen gas is likely to be a key player. The gas is a clean fuel: if it is used in a fuel cell to produce electricity, the only by-product is water. But it has one major drawback: when hydrogen mixes with air, it becomes highly flammable. Leaks have to be detected quickly, so the need for hydrogen gas sensors will rise dramatically in the future. This has become a central topic in Langhammer’s research, although, in fact, he is mostly engaged in fundamental research in nanomaterials science.
“Over the past year interest in hydrogen has soared, not least in the EU, and more and more people are starting to realize that current hydrogen sensors are not good enough – and that sensors of this kind will be needed everywhere,” Langhammer says.
His work is localized in a research area called nanoplasmonics, which exploits the ability of light to interact with metallic nanoparticles. The light causes electrons to oscillate, creating plasmon resonance, which in turn can be used in sensors, for instance.
Gold is by far the most-used metal in this field, but Langhammer’s research team searched for alternatives, and managed to create nanoparticles from alloys of palladium and other noble metals. Palladium is good at absorbing hydrogen. When hydrogen atoms penetrate the particle, its volume and electronic structure change slightly, as does the plasmon resonance. This signals that hydrogen is present essentially as a small change in the color of the particle.
Grain boundaries allow hydrogen to move faster
The palladium sensor project was a great success. But Langhammer also discovered something else, which he now intends to study using the extended grant he has received as a Wallenberg Academy Fellow.
“This grant gives me the chance to remain at the international forefront of my research field – to be someone who others are inspired by. It’s fantastic.”
The alloyed nanoparticles consist of numerous minute crystallites, which also are known as grains. At the interface between such grains the material exhibits special properties. It is already known that conductivity, for example, is different at the grain boundaries. But Langhammer and his colleagues saw something else.
“We discovered that hydrogen diffuses extremely quickly at the grain boundaries – faster than in the rest of the crystal. Now I want to see how the boundaries affect hydrogen absorption in nanoparticles and how the boundary structure is affected by particle composition. I hope that by optimizing the grain boundaries of the particles, we will eventually be able to make even faster sensors.”
The grain boundaries in a material can also change spontaneously, potentially worsening a sensor’s performance over time. For this reason, Langhammer wants to find methods of stabilizing the grain boundaries in their optimal state.
“Grain structure is also important in metallurgy, where a wide range of products, from steel to high-temperature alloys, are made. We know from that field that if you mix in small quantities of other elements, they tend to migrate to the grain boundaries and block them, thereby stabilizing the grain structure. I want to try this out with our nanoparticles.”
Using methods that Langhammer and his colleagues have themselves devised, they can fabricate one nanoparticle at a time with the utmost precision, and change the quantity of substances involved by a couple of percent at a time, as they seek to find the perfect balance.
But a materials scientist also needs to consider sustainability. Are the materials toxic, uncommon, costly?
“The materials we use do include gold and palladium. They’re noble metals, so they’re not exactly the cheapest options around. But it’s the unique properties of palladium interacting with hydrogen that makes it so suitable, so we can’t really get around it. One advantage of our method, however, is that only incredibly small quantities of material are needed for effective sensors.”
From ideas to reality in just a few years
Langhammer came to Sweden from Switzerland to write an engineering dissertation, and stayed. In parallel with his work in the academic world, he has co-founded two companies: one that commercializes sensors, and one engaged in microscopy technology and nanofluidics. He started the sensor company with his doctoral supervisor and with his wife, who was also researching at Chalmers University of Technology. She stayed with the company, and he very nearly did too. But he gradually grew into the role of research leader, and has seen it evolve in recent years.
“I have a much larger team now, and don’t have as much time for each individual colleague. This means I have to give a great deal of responsibility to the people working with me. I felt some trepidation to start with, given how important it is that things go well, but in fact it’s been wonderful to see how people have grown as a result.”
When he looks back, he sees how things that were perceived to be wild ideas and high-risk projects just a few years ago are now a reality.
“The next step is to revitalize our research to attract next generation funding. Also, six months ago I was appointed co-leader of the Chalmers’ Excellence Initiative Nano. So new challenges constantly arise. And I’m still working quite a lot with the companies. Priorities there are completely different than those in academia, and it sometimes feels a bit like a refuge. It’s great to have the best of both worlds.”
Text Lisa Kirsebom
Translation Maxwell Arding
Photo Anna-Lena Lundqvist, Magnus Bergström, Yen Strandqvist