What damages the kidneys during inflammation and bacterial infections? Diana Karpman studies mechanisms of disease with the goal to prevent kidney injury and to spare patients the need for dialysis and transplants.
Diana Karpman
Chief physician in pediatric nephrology and Professor of Pediatrics
Wallenberg Clinical Scholar 2016
Institution:
Lund University
Research field:
Mechanisms of renal damage in infection and inflammation
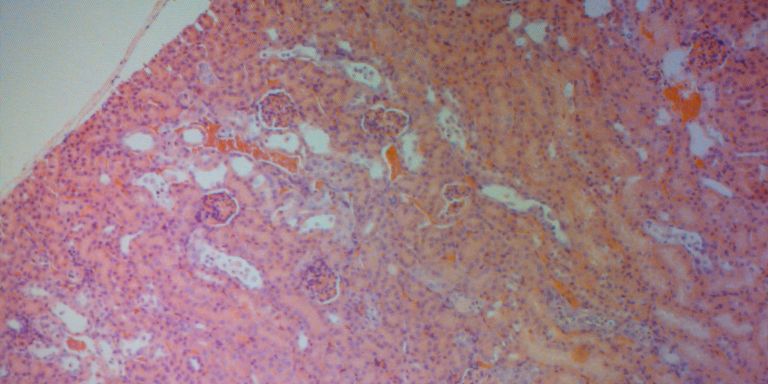
Diana Karpman had just completed her medical studies and was on call at a regional hospital one Christmas Eve when a very sick child was admitted. The young girl had an EHEC infection, a potentially life-threatening type of E. coli bacteria, and developed renal failure. In some patients this leads to severe kidney damage requiring dialysis or a kidney transplant.
“I felt there should have been a way to save the child’s kidneys. I thought there was something I simply didn’t understand yet,” Diana Karpman recalls.
This lead to her to read about EHEC infections and kidney failure. After having read hundreds of scientific articles, she concluded that no one actually knew how bacterial toxins damage the kidneys. Diana Karpman decided to carry out research on this.
Microvesicles in focus
She is now heading a team of some 15 researchers, one of the largest in the Nordic region specializing in research of kidney diseases. She also established the section of pediatric nephrology at Skåne University Hospital, which treats over one thousand patients per year.
As a Wallenberg Clinical Scholar, Diana Karpman will continue to study mechanisms of kidney disease, with the ultimate goal of preventing kidney damage. This could save the kidneys of many patients.
A central aspect of the research is microvesicles. These are small membrane particles that detach from cells and bear cellular content within and on their membranes. This is a physiological mechanism by which cells transport substances, and probably also a means to rid cells of harmful content.
“Being chosen to be a Wallenberg Clinical Scholar means a lot to me! It’s an honor, a fantastic grant allowing me to pursue the line of research I consider promising. It is also a confirmation that someone else thinks that we are on the right track.”
Toxins and blood clots in the kidneys
During EHEC infection bacteria colonize the intestine and release a toxin termed Shiga toxin. This toxin damages the intestinal lining and affects blood vessels as well as blood cells, mainly platelets.
“When the toxin attaches to platelets, they shed microvesicles containing the toxin, and other inflammatory substances. The release of microvesicles is most likely a cell-protective mechanism which, unfortunately, affects other cells,” Diana Karpman explains.
Her research has shown that the toxin and the inflammatory substances are transported to the kidneys by microvesicles. There microvesicles are taken up by kidney cells, and induce cell death and inflammation. In addition, toxin-bearing platelets aggregate, leading to small blood clots harmful to the circulation within the kidneys.
Diana Karpman’s research team aims to study mechanism by which microvesicle uptake can be prevented.
Microvesicles can promote inflammation
Cells within renal blood vessels also release microvesicles. Diana Karpman’s group has a perfusion system that mimics the blood flow within blood vessels and is coated with endothelial cells, the cells present on the inner lining of blood vessels.
Blood or plasma is perfused at a predetermined flow rate allowing the group to study what happens the cells. When testing plasma from patients with vasculitis (a form of vascular inflammation of the kidneys), the group found that patient plasma caused the cells to release microvesicles. This induced and worsened the inflammation.
This is due to the presence of surface molecules on the microvesicles that can activate the host response and exacerbate inflammation. These molecules can also detach from the circulating microvesicles and thereby be transferred to other cells. This results in the transfer of inflammation from one area to another.
“We have now found a way to block the release of microvesicles. This may potentially be developed into a treatment for vascular inflammation”, says Diana Karpman.
Inspired by patient contact
In addition to the study of microvesicles, Diana Karpman carries out research on other mechanisms that cause inflammation and blood aggregates in the kidneys.
One week every month she works at the hospital clinic treating children with kidney disease. There, she has the same clinical routines as the other doctors. Patient contact inspires new research ideas. Several of the fellow researchers in her team also work in the clinic, and when it is time for the research team’s weekly lab meeting, they walk from the clinic to the laboratory along the glass walkways that link the buildings at Lund university hospital.
“Half of the group attends lab meetings in health-care uniforms, half in civilian clothes. Close contact with patients is essential. We are aware of the current patients treated at the clinic and can identify patients relevant to our research. In addition, we have access to patient samples.”
Even if Diana Karpman’s expertise is pediatric kidney disease, many of the diseases she treats are just as relevant to adults.
“We study pediatric diseases simply because those are the patients we have. Of course I hope our findings will ultimately benefit as many patients as possible.”
Text Lisa Kirsebom
Translation Maxwell Arding
Photo Magnus Bergström