Project Grant 2017
Development of new therapeutic strategies based on the discovery of ZC3H11A
Principal investigator:
Leif Andersson, Professor of Functional Genomics
Institution:
Uppsala University
Grant in SEK:
SEK 43 million over five years
SEK 18 million in 2017
SEK 25 million in 2020
There are at least 600 known viruses that can infect humans. The body can defend itself against some of them fairly easily. We have learnt to fight others using vaccines or antiviral preparations. Some of them continue to confound us, causing chronic symptoms; a few are guaranteed killers.
In the ongoing coronavirus pandemic, viruses are a daily topic of conversation, but viruses were actually not discovered until the 1930s, with the advent of the electron microscope. The SARS-coV-2 virus has increased the importance of finding new antiviral treatments as well as vaccines. Those antiviral treatments are important because they can be used to treat people who are already infected.
Leif Andersson and his research team are on the trail of a gene that suppresses the growth of some important viruses that cause pain and suffering, and that, untreated, may lead to death: adenovirus, influenza virus, herpes virus and HIV. The same gene – ZC3H11A – also seems to be able to influence tumor growth, at least in mice.
“You might say that we are pursuing two lines of research in the project: first, to understand how ZC3 – as we call the gene for short – affects viral replication; and second, how it affects tumor growth,” says Andersson, who is a professor of functional genomics at Uppsala University.
Pigs to thank for discovery
The first pieces in the puzzle were laid by Andersson and his colleagues as far back as the late 1980s. At that time they were mapping pig DNA and the genetic differences between wild boar and domestic pigs. The largest and most numerous pieces in the puzzle were identified later, when the first sequencing of the human genome was presented in 2000.
“It was a milestone in genetics. Many of the 20,000 protein-coding genes have been studied exhaustively, and we know the diseases that are associated with changes in them. But we still know little about the exact functions of many genes in the body and in cells.
In 2003 Andersson’s research team published an article in Nature showing how the interaction between an unknown protein and the gene for a growth factor played a key role in regulating muscle growth in pigs. A few years later they were also able to identify a previously unknown “zinc finger” protein, which they named ZBED6.
“It had been overlooked when the human genome was presented. It was located inside another gene – ZC3H11A – like a Russian Matryoshka doll. It was then that we began to ponder the significance of ZC3.”
They decided to inactivate the gene by removing it using the Crispr-Cas9 gene editing tool, and see what happened.
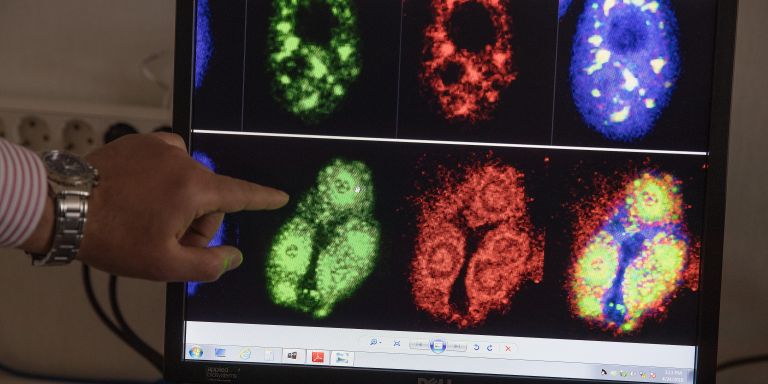
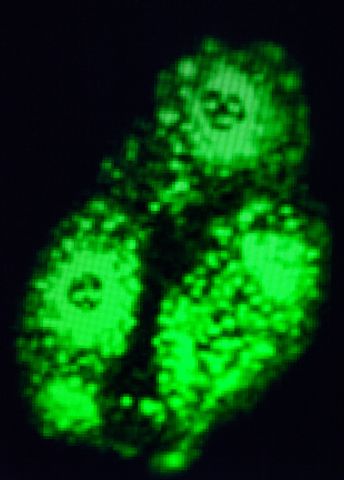
“Not a lot happened. But when we tried to stress the cell by infecting it with a virus, something unexpected happened – the virus had problems replicating in the cell nucleus. The number of virus particles declined dramatically.”
Important function
Not all, but a good number of medically important viruses replicate in the cell nucleus. Adenovirus, influenza virus, herpes virus and HIV are among them.
“We found that transmission of the viral DNA from the cell nucleus to the cytoplasm is disrupted. This offers a window for controlling how ZC3 impacts viruses, and potential for developing an antiviral drug.”
But Andersson does not think this discovery has any implications for the fight against coronavirus.
“We’ll naturally be looking into it, but we believe it to be unlikely, since coronavirus does not replicate in the cell nucleus. But what we have learnt over the years is that the protein appears to have more functions. In addition to affecting the way the cell deals with stress, it seems to play a part in regulation of the immune system. Improved understanding of this may be even more important than the transport of viral mRNA.”
This is in turn linked to the cancer part of their research. Cancer tumors are stressed; they require nutrients and blood supply to multiply.
“ZC3H11A is a stress-related protein. It’s important for the immune system to maintain a balance between stepping on the gas and braking. This is directly related to immunotherapy for cancer. We don’t know exactly what role ZC3 plays in regulating the immune system, directly or indirectly, depending on which proteins are expelled from the cell nucleus under stress. But we can already say there is a link between ZC3’s function and the development of certain tumors.”
But for the research team, the fundamental goal is to understand the function of ZC3, where it is located in the cell, and the proteins with which it interacts.
“Before we made our study, very little was known. We have seen that ZC3 is present in virtually all cells, which suggests that it performs a basic function in mammalian cells. If we inactivate the ZC3 gene in a mouse fetus, it will not be born. This indicates that it also has a key function during fetal development.”
It is hard to say whether the project, which is being funded by Knut and Alice Wallenberg Foundation, will lead to an antiviral drug and a new type of cancer therapy.
“This is pure scientific research. Industry will then have to take over to see if our findings can be translated into a successful therapy. It’s always hard to know whether or not development of a given drug will be successful,” Andersson concludes.
Text Carina Dahlberg
Translation Maxwell Arding
Photo Mikael Wallerstedt, Magnus Bergström